
Cuban Journal of Forest Sciences. 2022; May-August 10(2): 197-214
Translated from the original in spanish
Original article
Differential response in the acicular anatomy of Pinus caribaea var. Caribaea and Pinus tropicalis (Pinaceae) in sympatry
Respuesta diferencial en la anatomía acícular de Pinus caribaea var. caribaea y Pinus tropicalis (Pinaceae) en simpatría
Resposta diferencial na anatomia acicular de Pinus caribaea var. caribaea e Pinus tropicalis (Pinaceae) em simpatria
Gretel Geada López1*![]() https://orcid.org/0000-0002-8421-0624
https://orcid.org/0000-0002-8421-0624
Luitmila Pérez-del Valle1![]() https://orcid.org/0000-0002-1838-4482
https://orcid.org/0000-0002-1838-4482
Rogelio Sotolongo-Sospedra1![]() https://orcid.org/0000-0003-0116-4157
https://orcid.org/0000-0003-0116-4157
1University of Pinar del Río "Hermanos Saíz Montes de Oca". Pinar del Rio, Cuba.![]()
*Corresponding author: gabriel@upr.edu.cu
Received:06/13/2022.
Approved:06/29/2022.
ABSTRACT
Pinus caribaea var. caribaea and Pinus tropicalis are the two species of the genus Pinus that form the pine forests of western Cuba, either in pure stands or in sympatry. The objective of this study is to evaluate the differential response in foliar anatomy of both species in the same ecotopes. Cross sections of the needles were made and 12 anatomical variables were measured. The statistical analyzes used, allowed to clearly differentiate the two species, each one presents its own variations to adapt to the same environment. The Mann-Whitney test showed that there are differences in nine of the 12 anatomical variables between the two species in each ecotope and only those variables related to photosynthesis and transport were similar between species. Discriminant analysis showed that each population of the same species is ordered in the opposite way with respect to the other. The variables that contribute to the discrimination between the species are the number of stomata, thickness of the epidermis, thickness of the cuticle and number of resin channels. Both species present a differential response in their anatomical variables to similar ecotope conditions that are adaptive responses and not anatomical adjustments.
Keywords: Leaf anatomical variation; Differential response; Pinus tropicalis; Sympatry.
RESUMEN
Pinus caribaea var. caribaea y Pinus tropicalis son las dos especies del género Pinus que forman los pinares del occidente de Cuba ya sea en masas puras o en simpatría. El objetivo de este trabajo es evaluar la respuesta diferencial en anatomía foliar de ambas especies en iguales ecótopos. Se realizaron cortes transversales de las acículas y se midieron 12 variables anatómicas. Los análisis estadísticos empleados permitieron diferenciar claramente las dos especies, cada una presenta variaciones propias para adaptarse a un mismo ambiente. La prueba de Mann-Whitney mostró que existen diferencias en nueve de las 12 variables anatómicas entre las dos especies en cada ecótopo y solo aquellas variables relacionadas con la fotosíntesis y el transporte fueron similares entre especies. El análisis de discriminantes mostró que cada población de la misma especie se ordena de forma opuesta con respecto a la otra. Las variables que contribuyen a la discriminación entre las especies son el número de estomas, grosor de la epidermis, grosor de la cutícula y número de canales de resina. Ambas especies presenta una respuesta diferencial en sus variables anatómicas a similares condiciones del ecótopo que son respuestas adaptativas y no de ajustes anatómicos.
Palabras clave: Variación anatómica foliar; respuesta diferencial; Pinus tropicalis; Simpatría.
RESUMO
Pinus caribaea var. caribaea e Pinus tropicalis são as duas espécies do gênero Pinus que formam os pinhais do oeste de Cuba, seja em povoamentos puros ou em simpatria. O objetivo deste trabalho é avaliar a resposta diferencial na anatomia foliar de ambas as espécies em um mesmo ecótopo. Cortes transversais das agulhas foram feitos e 12 variáveis anatômicas foram medidas. As análises estatísticas utilizadas permitiram diferenciar claramente as duas espécies, cada uma apresenta suas próprias variações para se adaptar ao mesmo ambiente. O teste de Mann-Whitney mostrou que existem diferenças em nove das 12 variáveis anatômicas entre as duas espécies em cada ecótopo e apenas as variáveis relacionadas à fotossíntese e transporte foram semelhantes entre as espécies. A análise discriminante mostrou que cada população da mesma espécie é ordenada de forma oposta em relação à outra. As variáveis que contribuem para a discriminação entre as espécies são o número de estômatos, espessura da epiderme, espessura da cutícula e número de canais de resina. Ambas as espécies apresentam uma resposta diferencial em suas variáveis anatômicas a condições semelhantes de ecótopos que são respostas adaptativas e não ajustes anatômicos.
Palavras-chave: Variação anatômica foliar; Resposta diferencial; Pinus tropicalis, Simpatria.
INTRODUCTION
Pines in Cuba are distributed in extreme edaphic conditions, either due to the unfavorable physical-chemical characteristics of the substrate or due to orographic aspects (Samek and Del Risco-Rodríguez 1989; Farjon and Filter 2013). These very nutrient-poor soil habitats are accompanied, in many cases, by low water availability and recurrent fire regimes, which implies low competition with angiosperms (Keeley 2012; Badik et al., 2018) and the formation of continuous masses where they constitute the dominant species (Samek and Del Risco-Rodríguez 1989).
In western Cuba, this formation occupies about 50 % of the forest area, concentrated in Pinar del Río and Isla de la Juventud and represented by Pinus tropicalis Morelet (tropical pine) and Pinus caribaea Morelet var. caribaea Barret and Golfari (caribbean pine).
P. tropicalis is a Cuban endemism of subg. Pinus subsect. Pinus in America (Geada-López et al., 2004; Gernandt et al., 2005), forms continuous monotypic pine forests in Pinar del Rio and the center of La Isla de la Juventud on oligotrophic substrates of slate, sandstone and quartzite sands (Samek and Del Risco-Rodriguez 1989). P. caribaea var. caribaea, on the other hand, can occupy areas sympatrically with P. tropicalis and only naturally forms pure masses on substrates derived from serpentine and ultrabasic rocks on the Cajálbana plateau (López-Almirall 1982; Samek and Del Risco-Rodríguez 1989; Farjon and Filter 2013).). From an evolutionary point of view, it is a recent species and its varieties differ from both a morphological and genetic point of view (Rebolledo-Camacho et al., 2018).
Variations in the morphology and anatomical structure of the needles between individuals and populations are due to differences in the soil conditions and the humidity regimes of the habitat where they grow (Tiwari et al., 2013; Ghimire et al., 2014; Meng et al., 2018) and can be used as a rapid method to explore morphoanatomical variability between populations (Boratyñska et al., 2015; Zhang et al., 2017). Changes in the dimensions of needle tissues have been documented for species with continental distribution ranges (Boratyñska et al., 2015; Jankowski et al., 2017; 2019, Köbölkuti et al., 2017) and contrasting environments (Boratyñska et al., 2015; Hodžiæ et al., 2020). However, studies like these on island species with a small distribution and apparently homogeneous climatic conditions are scarce (Pérez-del Valle et al. 2020) and only one report in Cuba on P. caribaea var. caribaea by Geada-López et al., 2021).
On the other hand, it would be possible to expect in phylogenetically close species, within the subgenus Pinus and living in sympatry, a similar behavior in their foliar tissues in response to ecotope conditions during their adaptation. The objective of this study was to evaluate the anatomical response of both species in natural populations and in sympatry. Since, for success in management programs, mainly in the design of conservation strategies, it is necessary to start from the knowledge of genetic variability, phenotypic plasticity and genetic differences in the plasticity of forest species in adaptive characters, between and within their populations.
MATERIALS AND METHODS
Sampling
The samples were taken in natural pine forests where Pinus caribaea var. caribaea (this species will be treated from now on as Pinus caribaea) and Pinus tropicalis, in localities of the province of Pinar del Río and Artemisa, Cuba, where they live in sympatry (Figure 1). These localities represent the majority of the habitats in which these taxa are present and are defined by their formation and lithology, the altitudinal floor and the slope according to Geada-López et al., (2021). The combination of these three characteristics is the basis for identifying the ecotopes (Table 1), the localities of Marbajita and San Ubaldo represent sites where pure masses of Pinus caribaea and P. tropicalis are found, respectively.
Table 1. - Georeferencing and ecotopes characteristics
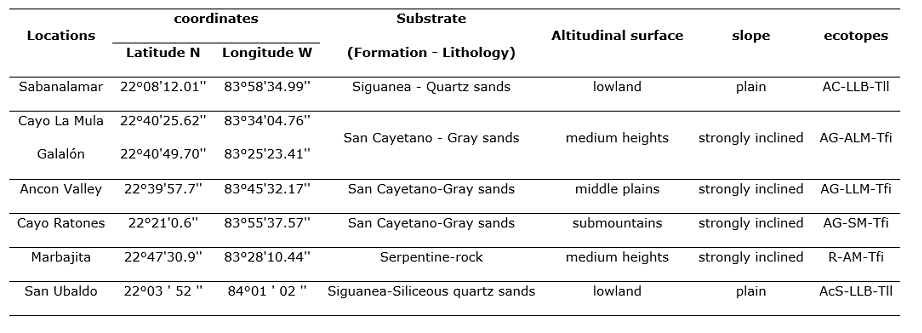
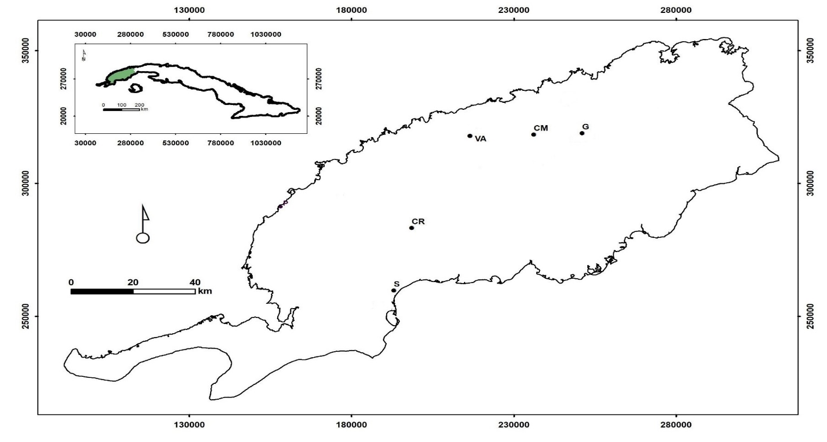
Figure 1- Geographical distribution of studied localities
B: Sabanalamar, CR: Cayo Ratones, CM: Cayo la Mula, G: Galalón, VA: Valle Ancón
Measurements of traits in the needles
In each locality, needles were collected from the lower ⅔ of the crown of 30 randomly selected individuals in the adult stage. For the anatomical study, 10 needles were chosen at random from each individual sampled in each locality. Cross sections were made in the middle part of the needle for observations and measurements with an NLCD-307B optical microscope. All cuts were made by hand on fresh tissue and temporary preparations.
The anatomical variables measured at 400× magnification were: Thickness of the cuticle (GC), Thickness of the epidermis (GEp), Thickness of hypodermis (GH), and counted the number of hypodermal cell layers. (CH). At 100× magnification, the thickness of the transfusion tissue (PT), the thickness of the chlorophyllic parenchyma (CP), the diameter of the left resin canal (DCi), the diameter of the right resin canal (DCd), the height of the vascular bundle (HHv) and the width of the vascular bundle (AHv) and counted, with this lens the number of stomata (NoE), the number of resin canals (NC). All variables were defined according to Pérez-del Valle et al. (2020).
Statistic analysis
For the analysis, only the ecotopes of the localities where the species inhabit in sympatry were considered. The behavior of the anatomical variables between species was compared using the inference test based on two Mann-Whitney (U) samples (p <0.05). For the comparison between species in each ecotope where they live in sympatry (AC-LLB-Tll in Sabanalamar, AG-ALM-Tfi in Cayo La Mula and Galalón, AG-LLM-Tfi in Valle Ancón and AG-SM-Tfi in Cayo Ratones) the same test was used.
A diagram was made according to the methodology of Jentys-Szaferowa (1959) to distinguish the variation in anatomy between the ecotopes studied and of these with respect to the standard line for the species, which is assumed as the averages of each variable, represented in the diagram by a line through the value of one.
To maximize the differences between the two species and distinguishing the variable or variables that most contribute to differentiating them, a discriminant analysis was carried out, as a priori groups the observations of the anatomical variables were considered by the species-ecotope combinations. Localities where both species form pure stands were included in this analysis. Statistical analyzes were performed with the program Infostat ver. 15 (DiRienzo et al., 2015).
RESULTS
According to the results of the Mann-Whitney Test, P. caribaea and P. tropicalis differs significantly in most of the anatomical variables analyzed, especially the greatest differences are observed in the number of canals (NC), the thickness of the transfusion tissue (PT) and the thickness of the cuticle (CG) while the variables thickness of the chlorophyllic parenchyma (PC) and height and width of the conduction tissue (HHv and AHv) did not differ significantly (Table 2 and Figure 2).
Table 2. - Mean values and standard deviation of the anatomical variables evaluated for
Pinus caribaea
and Pinus tropicalis and the statistics of the Mann-Whitney (U) test
(a=0.05)
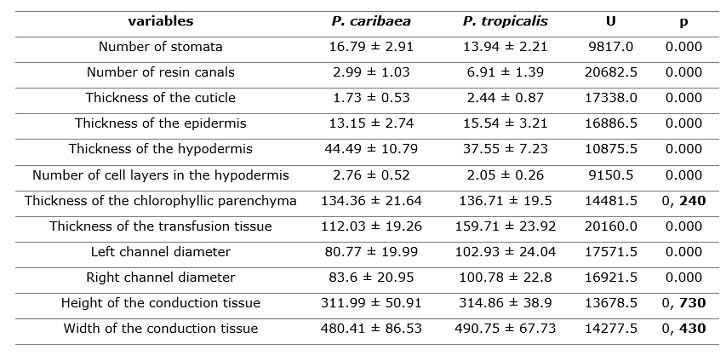
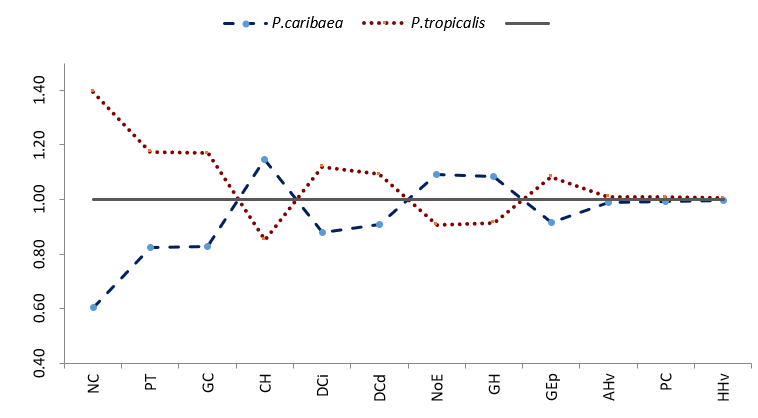
Figure 2. - Jentys-Szaferowa diagram of the anatomical variability of the needles of P. caribaea and P. tropicalis
GC: Thickness of the cuticle, GEp: Thickness of the epidermis, GH: Thickness of the hypodermis, CH: Number of hypodermis cell layers, NoE: Number of stomata, PT: Thickness of transfusion tissue, NC: Number of the resin canals, PC: Thickness of chlorophyllic parenchyma, DCi: Diameter of the left canals, DCd: Diameter of the right canal, HHv: Height of the conduction tissue, AHv: Width of the conduction tissue
Table 3. - Mean values and standard deviation of the anatomical variables of
P. caribaea and P. tropicalis in the ecotopes
in sympatry and the statistics of the Mann-Whitney (U) test
(a= 0.05)
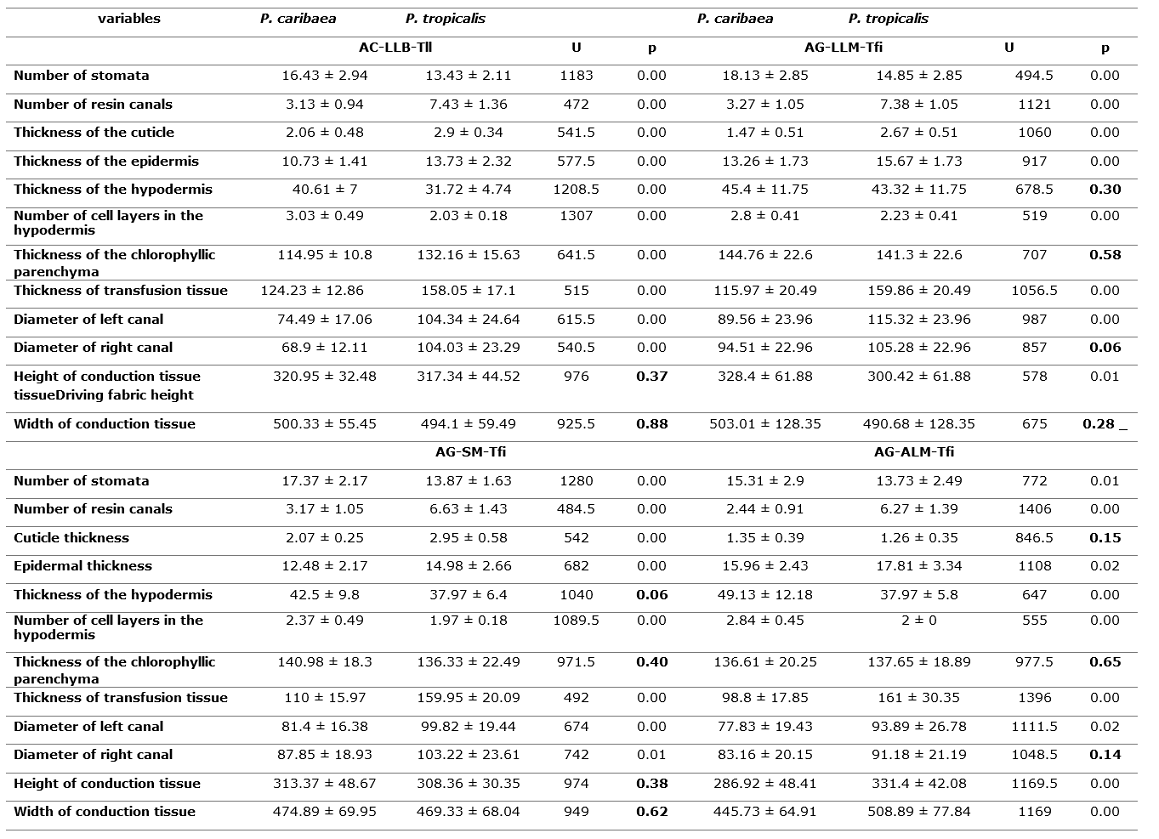
AC-LLB-Tfi: Quartz sands-lowland-plain, AG-LLM-Tfi: Gray sands-middle plains-strongly inclined, AG-SM-Tfi: Gray sands-submountains-strongly inclined, AG-ALM- Tfi: Gray sands:medium heights:strongly inclined
There is a differential response in the leaf anatomy of each species for the same ecotope (Table 3 and Figure 3), fundamentally in variables such as the number of stomata (NoE), the number of canals (NC), the thickness of the epidermis (GEp), the number of hypodermal cell layers (CH), the thickness of the transfusion tissue (PT), all of which are related to water regulation. This analysis shows that several of these anatomical features and differences between the two species are independent of environmental conditions. However, there are variables that have responses associated with the characteristics of the ecotope, such as the thickness of the chlorophyll parenchyma (CP), which does not differ statistically in the ecotopes whose lithology is gray sands (Figure 3).
The Jentys-Szaferowa diagrams clearly confirm the variability of the two species in the four ecotopes and their differences even in sympatry.
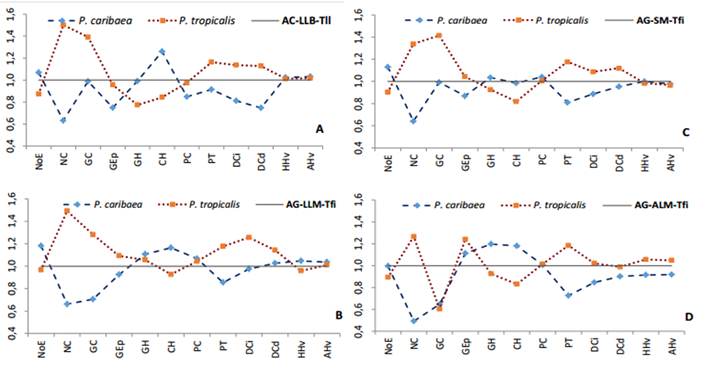
Figure 3. - Jentys-Szaferowa diagram of the anatomical variability of
Pinus caribaea var. Caribbean and
Pinus tropicalis in ecotopes in sympatry.
A: Quartzite sandstones-low plains-flat terrains,
B: Gray sandstones-medium plains-strongly inclined terrains,
C: Gray sandstones-submountains-strongly inclined terrains,
D: Gray sandstones-medium heights-strongly
inclined terrains
GC: Cuticle thickness, GEp: Epidermis thickness, GH: Hypodermis thickness, CH: Number of hypodermis cell layers, NoE: Number of stomata, PT: Transfusion tissue thickness, NC: Number of channels, PC: Thickness of the chlorophyll parenchyma, DCi: Diameter of the left channel, DCd: Diameter of the right channel, HHv: Height of the conduction tissue, AHv: Width of the conduction tissue.
Discriminant analysis
Table 4 presents the results of the discriminant analysis; the first two functions explain 88.15 % of the variations between species by ecotope. According to the standardized coefficients, the most important variables in the discrimination in the first function are the number of stomata and canals and the thickness of the cuticle and the epidermis in the second.
According to the values of the centroids (Table 4) the first function maximizes the differences between the two species, P. tropicalis is located towards the negative end of canonical axis 1 and towards the positive end P. caribaea in both cases regardless of the ecotope (Figure 4). According to the two variables with the greatest weight in this function and the sign, a greater number of stomata (NE) in P. caribaea and a greater number of canals (NC) in P. tropicalis are the variables that most discriminate both species.
The second function suggests a gradient in the location of the species according to the characteristics of the ecotope, the variables thickness of the cuticle and the epidermis oppose in the case of P. tropicalis the ecotope of AG-ALM-Tfi, located towards the negative part of the axis, to the other three arranged in the positive part, and in the case of P. caribaea the ecotopes AG-ALM-Tfi and AG-LLM-Tfi are located towards the negative part of the axis and AC-LLB-Tll and AG-SM-Tfi towards the positive of this (Figure 4 and 5).
Table 4. - Results of the discriminant analysis, standardized coefficients of each variable and centroids of the ecotopes in sympatry in the two discriminant functions
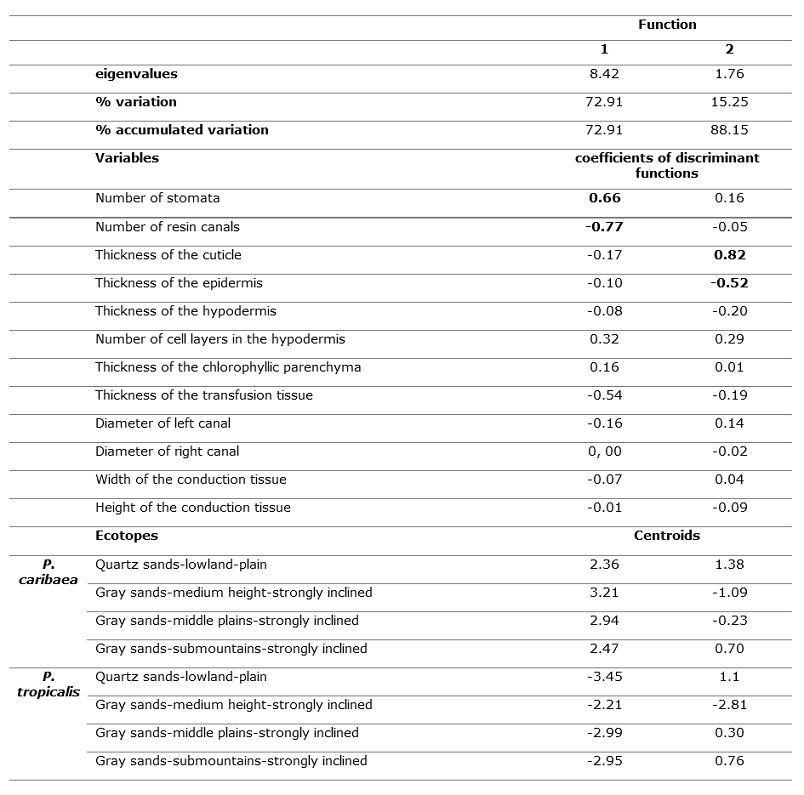
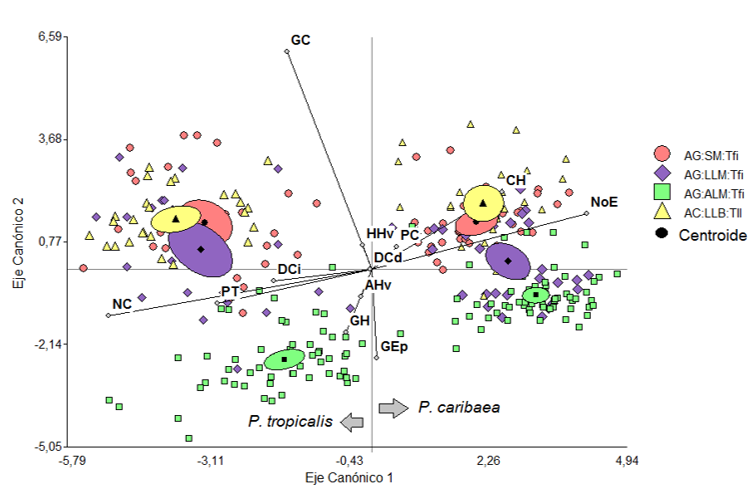
Figure 4 - Ordering of the species P. caribaea
and P. tropicalis in the ecotopes that inhabit
in sympatry according
to the first two discriminant functions and based on anatomical
variables of the needles
GC: Cuticle thickness, GEp: Epidermis thickness, GH: Hypodermis thickness, CH: Number
of hypodermis cell layers, NoE: Number of stomata, PT: Transfusion tissue thickness, NC: Number
of channels, PC: Thickness of the chlorophyllic parenchyma, DCi: Diameter of the left channel,
DCd: Diameter of the right channel, HHv: Height of the conduction tissue, AHv: Width of the
conduction tissue
Ecotopes: AC-LLB-Tfi: Quartz sands-lowland-plain, AG-LLM-Tfi: Gray sands-middle
plains-strongly inclined, AG-SM-Tfi: Gray sands-submountains-strongly inclined, AG- ALM-Tfi: Gray
sands-medium heights-strongly inclined.
Likewise, when the two localities where the species form pure masses are included in the analysis, each species is located in the plane of the discriminant function in an opposite way and in similar positions to the ecotopes where they live in sympatry (Figure 5). Therefore, the behavior of anatomical variation is a characteristic of the species.
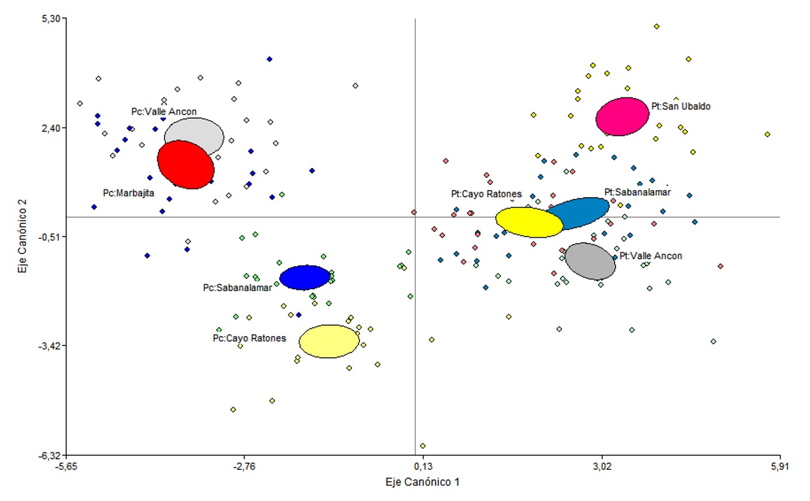
Figure 5. - Ordering of the species P. caribaea
and P. tropicalis in the ecotopes that inhabit
in sympatry and in pure stands according
to the first two discriminant functions and based
on anatomical variables of the needles
DISCUSSION
The study clearly shows the anatomical differences between the two species (Table 2, 3, Figure 2 and 3). Each species has developed its own anatomical and functional mechanisms to counteract the same edaphic conditions. These species in common reinforce the structure of the epidermal and hypodermal tissues, both the thickness of the cuticle and the presence of sclereids in the hypodermis. These aspects are reported as adaptations of the genus to very extreme environments (Dörken and Stützel 2012; Grill et al., 2004). Those features are common to both species. The difference between both species lies in the fact that P. caribaea develops a greater number of layers and thickness of the hypodermis (multiform type) as an adaptation to the same conditions. On the other hand, P. tropicalis develops a somewhat thicker cuticle accompanied by a greater number of sclereids. This behavior in the structure of P. tropicalis was first reported for the species by Pérez-del Valle et al. (2020) and is similar to what is documented in species of the subsect. Pinus such as: P. taboliformis (Zhang et al., 2017), P. thunbergii (Ghimere et al., 2014), P. roxburghii (Tiwari et al., 2013), or species of its section that inhabit very dry environments: P. canariensis (Grill et al., 2004), P. heldreichii (Nicholiæ et al., 2016).
The presence of mechanical tissue (sclerenchyma) in the hypodermis and between the vascular bundles in Pinus tropicalis contributes to increasing resistance to edaphic drought and conferring rigidity to the long needles in the species. Similar adaptations and decrease in the number of stomata were reported in P. canariensis (Grill et al., 2004; López et al., 2010), P. tabuliformis (Meng et al., 2018) and P. sylvestris (Köbölkuti et al., 2017) to face very dry environments. This element seems to be related to the water economy in Asian species, in particular the decrease in the number of stomata and their density compared to species of Pinus subsect. Trifoliae as is the case of P. cariabea (Tiwari et al., 2013; Donnelly et al., 2016; Galdina and Khazova 2019).
The results of wide and numerous resin canals of P. tropicalis on P. caribaea in any ecotope confirm the productivity of the first on the second species (Table 3). These characteristics in the channels of P. tropicalis compared to P. caribaea although it was documented by López-Almirall and Albert-Puente (1982) in a foliar anatomical study to detect differences between the four Cuban species and later recognized by Pérez del Valle et al. (2020) in a comparative anatomical study, precise conclusions could not be reached due to the nature of the study and sampling limitations.
López-Almirall (1982) recognized the great variability in the morphological and reproductive characteristics between pure natural stands of Pinus caribaea and the existence of slight differences between populations in P. tropicalis. Subsequently, Pérez-del Valle et al. (2020) documented the differences between both species when they inhabited two ecotopes sympatrically, but due to the descriptive purpose of the study, it did not allow to delve into the magnitude of the effect of the ecotope. In the present study, the differences between the ecotopes are more notable (Figures 4 and 5, Table 3), which demonstrates their great phenotypic plasticity, good competitive capacity in relation to P. tropicalis.
In addition, this result is consistent with the idea proposed by Pérez-del Valle et al. (2020) and Geada-López et al. (2021) about the origin of the differences between provenances within the trial of these for the species P. caribaea (García-Quintana et al., 2007) in relation to the same trials in P. tropicalis (Mercadet-Portillo et al., 2001). This is also related to the great differences in leaf anatomical structure depending on the substrate, which are more evident in P. caribaea (Figure 4 and 5).
Both the mean comparison analysis and the classification analysis (Table 2, 4, Figures 4 and 5) show that the substrate has a marked influence on the anatomy of the needles by differentiating each ecotope within each species. Thus, for example, the lithology of quartzite sands, which represent one of the most extreme ecotopes from the hydric point of view due to their high infiltration and very low retention of humidity, determine a more singular behavior in the anatomy.
On the other hand, in both species within their ecotopes and in particular for Pinus tropicalis, the stability in the anatomical characteristics suggests the existence of a longer-term adaptation in the species (López-Almirall 1982; Geada-López 2003). Although this marked effect of the ecotope on leaf anatomy in the genus is recognized, it has been documented for species with wide distribution ranges (Zhang et al., 2017; Jankowski et al., 2017; Köbölkuti et al., 2017; Tyukavina et al., 2019). However, in species with a reduced distribution area and without great contrasts in climate and vegetation, they may not be subject to as much foliar variability. The results obtained reflect that, in insular species, especially Cuban ones, the conditions of the site or their geological origin cause them to display a phenotypic variation, which determines their adaptation to different ecotopes (Pérez-del Valle et al., 2020; Geada-López et al., 2021, 2022).
CONCLUSIONS
Pinus tropicalis and Pinus caribaea in sympatric association, each taxon has an opposite adaptive differential response in all its anatomical variables.
There is an effect of the ecotope on intraspecific anatomical variation in each species.
REFERENCES
BADIK, K., JAHNER, J. y WILSON, J., 2018. A biogeographic perspective on the evolution of fire syndromes in pine trees ( Pinus/ : Pinaceae). Royal Society Open Science [en línea], vol. 5, no. 3, pp. 172412. DOI 10.1098/rsos.172412. Disponible en: https://www.researchgate.net/publication/323909032_A_biogeographic_perspective_on_the_evolution_of_fire_syndromes_in_pine_trees_Pinus_Pinaceae.
BORATYÑSKA, K., SÊKIEWICZ, K., JASIÑSKA, A., TOMASZEWSKI, D., ISZKU£O, G., OK, T., BOU DAGHER KHARRAT, M. y BORATYÑSKI, A., 2015. Effect of geographic range discontinuity on taxonomic differentiation of Abies cilicica. Acta Societatis Botanicorum Poloniae [en línea], vol. 84, no. 4, pp. 419-430. DOI 10.5586/asbp.2015.037. Disponible en: https://www.researchgate.net/publication/288888569_Effect_of_geographic_range_discontinuity_on_taxonomic_differentiation_of_Abies_cilicica.
DONNELLY, K., CAVERS, S., COTTRELL, J. y ENNOS, R., 2016. Genetic variation for needle traits in Scots pine (Pinus sylvestris L.). Tree Genetics & Genomes [en línea], vol. 12, no. 3, pp. 40-50. DOI 10.1007/s11295-016-1000-4. Disponible en: https://www.researchgate.net/publication/301252411_Genetic_variation_for_needle_traits_in_Scots_pine_Pinus_sylvestris_L.
DÖRKEN, V. y STÜTZEL, T., 2012. Morphology, anatomy and vasculature of leaves in Pinus (Pinaceae) and its evolutionary meaning. Fuel and Energy Abstracts [en línea], vol. 207, no. 1, pp. 57-62. DOI 10.1016/j.flora.2011.10.004. Disponible en: https://www.researchgate.net/publication/241094109_Morphology_anatomy_and_vasculature_of_leaves_in_Pinus_Pinaceae_and_its_evolutionary_meaning.
FARJON, A. y FILER, D., 2013. An Atlas of the World's Conifers: An Analysis of their Distribution, Biogeography, Diversity and Conservation Status [en línea]. Leiden, Países Bajos: BRILL. ISBN 978-90-04-21181-0. Disponible en: https://books.google.com.cu/books/about/An_Atlas_of_the_World_s_Conifers.html?id=uUNWAgAAQBAJ&redir_esc=y.
GALDINA, T. y KHAZOVA, E., 2019. Adaptability of Pinus sylvestris L. to various environmental conditions. IOP Conference Series: Earth and Environmental Science [en línea], vol. 316, no. 1, pp. 012002. DOI 10.1088/1755-1315/316/1/012002. Disponible en: https://www.researchgate.net/publication/336007121_Adaptability_of_Pinus_sylvestris_L_to_various_environmental_conditions.
GARCÍA QUINTANA, Y., ÁLVAREZ BRITO, A.F. y GUÍZAR NOLAZCO, E., 2007. Ensayo de procedencias de Pinus caribaea var. caribaea en Alturas de Pizarras, Viñales, Pinar del Río, Cuba. Revista Chapingo Serie Ciencias Forestales y del Ambiente [en línea], vol. 13, no. 2, pp. 125-129. Disponible en: https://www.researchgate.net/publication/322369196_Ensayo_de_procedencias_de_Pinus_caribaea_var_caribaea_en_Alturas_de_Pizarras_Vinales_Pinar_del_Rio_Cuba.
GEADA LÓPEZ, G., 2004. Phylogeny of Diploxylon pines (subgenus Pinus). Forest Genetics [en línea], vol. 11, no. 3-4, pp. 213-221. Disponible en: https://www.researchgate.net/publication/270902683_PHYLOGENY_OF_DIPLOXYLON_PINES_SUBGENUS_PINUS.
GEADA LÓPEZ, G., SOTOLONGO SOSPEDRA, R., PÉREZ DEL VALLE, L. y RAMÍREZ HERNÁNDEZ, R., 2021. Diferenciación anatómica foliar en poblaciones naturales de Pinus caribaea var. caribaea (Pinaceae) en Pinar del Río y Artemisa, Cuba. Revista del Jardín Botánico Nacional [en línea], vol. 42, pp. 175-188. [Consulta: 8 julio 2022]. ISSN 2410-5546. Disponible en: http://www.rjbn.uh.cu/index.php/RJBN/article/view/552.
GEADA LÓPEZ, G., SOTOLONGO SOSPEDRA, R., PÉREZ DEL VALLE, L. 2022. Variación anatómica foliar en poblaciones naturales de Pinus tropicalis en Pinar del Río. Revista Jardín Botánico Nacional Universidad de la Habana, 43: en Imprenta.
GERNANDT, D., GEADA LÓPEZ, G., GARCÍA, S. y LISTON, A., 2005. Phylogeny and Classification of Pinus. Taxon [en línea], vol. 54, no. 1, pp. 29. DOI 10.2307/25065300. Disponible en: https://www.researchgate.net/publication/271407044_Phylogeny_and_Classification_of_Pinus.
GHIMIRE, B., KIM, M., LEE, J.-H. y HEO, K., 2014. Leaf anatomy of Pinus thunbergii Parl. (Pinaceae) collected from different regions of Korea. Korean Journal of Plant Taxonomy [en línea], vol. 44, no. 2, pp. 91-99. DOI 10.11110/kjpt.2014.44.2.91. Disponible en: https://www.researchgate.net/publication/274974550_Leaf_anatomy_of_Pinus_thunbergii_Parl_Pinaceae_collected_from_different_regions_of_Korea.
GRILL, D., TAUSZ, M., PÖLLINGER, U., JIMENEZ, M. y MORALES, D., 2004. Effects of drought on needle anatomy of Pinus canariensis. Flora - Morphology, Distribution, Functional Ecology of Plants [en línea], vol. 199, no. 2, pp. 85-89. DOI 10.1078/0367-2530-00137. Disponible en: https://www.researchgate.net/publication/242355832_Effects_of_drought_on_needle_anatomy_of_Pinus_canariensis.
HODŽIÆ, M.M., HAJRUDINOVIÆ, A., BOGUNIÆ, F., MARKU, V., BALLIAN, Dalibor, HODŽIÆ, M., HAJRUDINOVIÆ-BOGUNIÆ, A., BOGUNIÆ, F. y BALLIAN, D, 2020. Geographic variation of Pinus heldreichii Christ from the Western Balkans based on cone and seed morphology. Dendrobiology [en línea], vol. 84, pp. 81-93. DOI 10.12657/denbio.084.007. Disponible en: https://www.researchgate.net/publication/347467389_Geographic_variation_of_Pinus_heldreichii_Christ_from_the_Western_Balkans_based_on_cone_and_seed_morphology.
JANKOWSKI, A., WYKA, T., ¯YTKOWIAK, R., DANUSEVIÈIUS, D. y OLEKSYN, J., 2019. Does climate-related in situ variability of Scots pine (Pinus sylvestris L.) needles have a genetic basis? Evidence from common garden experiments. Tree physiology [en línea], vol. 39, no. 4, pp. 573-589. DOI 10.1093/treephys/tpy145. Disponible en: https://www.researchgate.net/publication/334655400_Does_climate -related_in_situ_variability_of_Scots_pine_Pinus_sylvestris_L_needles_have_a_genetic_basis_Evidence_from_common_garden_experiments.
JANKOWSKI, A., WYKA, T., ÝYTKOWIAK, R., NIHLGÅRD, B., REICH, P. y OLEKSYN, J., 2017. Cold adaptation drives variability in needle structure and anatomy in Pinus sylvestris L. along a 1900 km temperate boreal transect. Functional Ecology [en línea], vol. 31, no. 12. DOI 10.1111/1365-2435.12946. Disponible en: https://www.researchgate.net/publication/318727745_Cold_adaptation_drives_variability_in_needle_structure_and_anatomy_in_Pinus_sylvestris_L_along_a_1900_km_temperate -boreal_transect.
JENTYS-SZAFEROWA, J., 1959. A graphical method of comparing the shapes of plants. Review of the Polish Academy of Sciences [en línea], vol. 4, no. 1, pp. 9-38. Disponible en: https://www.worldcat.org/title/graphical-method-of-comparing-the-shapes-of-plants/oclc/717114482.
KEELEY, J., 2012. Ecology and evolution of pine life histories. Annals of Forest Science [en línea], vol. 69, no. 4. DOI 10.1007/s13595-012-0201-8. Disponible en: https://www.researchgate.net/publication/257805652_Ecology_and_evolution_of_pine_life_histories.
KÖBÖLKUTI, A., TOTH, E.G., LADÁNYI, M. y HÖHN, M., 2017. Morphological and anatomical differentiation in peripheral Pinus sylvestris L. populations from the Carpathian region. Dendrobiology [en línea], vol. 77, pp. 105-117. DOI 10.12657/denbio.077.009. Disponible en: https://www.researchgate.net/publication/316056946_Morphological_and_anatomical_differentiation_in_peripheral_Pinus_sylvestris_L_populations_from_the_Carpathian_region.
LÓPEZ ALMIRALL, A., 1982. Variabilidad del género Pinus (Coniferales: Pinaceae) en Cuba. En: Accepted: 2020-01-22T18:10:55Z, Acta Botánica Cubana [en línea], vol. 12, pp. 1-32. [Consulta: 9 julio 2022]. ISSN 0138-6824. Disponible en: http://repositorio.geotech.cu/jspui/handle/1234/3935.
LÓPEZ, R., CLIMENT, J. y GIL, L., 2010. Intraspecific variation and plasticity in growth and foliar morphology along a climate gradient in the Canary Island pine. Trees [en línea], vol. 24, no. 2, pp. 343-350. DOI 10.1007/s00468-009-0404-2. Disponible en: https://www.researchgate.net/publication/225547576_Intraspecific_variation_and_plasticity_in_growth_and_foliar_morphology_along_a_climate_gradient_in_the_Canary_Island_pine.
LÓPEZ-ALMIRALL, A. y ALBERT-PUENTE, D., 1982. Características anatómicas de las agujas en especies cubanas de Pinus. Ciencias Biológicas (La Habana) [en línea], no. 8, pp. 3-16. [Consulta: 9 julio 2022]. Disponible en: https://biblat.unam.mx/pt/revista/ciencias-biologicas-la-habana/9.
MENG, J., CHEN, X., HUANG, Y., WANG, L., XING, F. y LI, Y., 2018. Environmental contribution to needle variation among natural populations of Pinus tabuliformis. Journal of Forestry Research [en línea], vol. 30, no. 1, pp. 1311-1322. DOI 10.1007/s11676-018-0722-6. Disponible en: https://www.researchgate.net/publication/325808245_Environmental_contribution_to_needle_variation_among_natural_populations_of_Pinus_tabuliformis.
PÉREZ DEL VALLE, L., GEADA LÓPEZ, G. y SOTOLONGO SOSPEDRA, R., 2020. Anatomía foliar comparada de Pinus caribaea var. caribaea y P. tropicalis (Pinaceae) en asociación simpátrica. Revista del Jardín Botánico Nacional [en línea], vol. 41, pp. 163-174. [Consulta: 9 julio 2022]. ISSN 2410-5546. DOI 10.5281/10.5281/zenodo.4776247. Disponible en: http://www.rjbn.uh.cu/index.php/RJBN/article/view/527.
REBOLLEDO CAMACHO, V., JARDÓN BARBOLLA, L., RAMIREZ, I., VAZQUEZ LOBO, A., PIÑERO, D. y VALERIO, P., 2018. Genetic variation and dispersal patterns in three varieties of Pinus caribaea (Pinaceae) in the Caribbean Basin. Plant Ecology and Evolution [en línea], vol. 151, no. 1, pp. 61-76. DOI 10.5091/plecevo.2018.1343. Disponible en: https://www.researchgate.net/publication/324041810_Genetic_variation_and_dispersal_patterns_in_three_varieties_of_Pinus_caribaea_Pinaceae_in_the_Caribbean_Basin.
TWARI, S., KUMAR, P., YADAV, D. y CHAUHAN, D., 2013. Comparative morphological, epidermal, and anatomical studies of Pinus roxburghii needles at different altitudes in the North-West Indian Himalayas. Turkish Journal of Botany [en línea], vol. 37, no. 1, pp. 65-73. DOI 10.3906/bot-1110-1. Disponible en: https://www.researchgate.net/publication/266388558_Comparative_morphological_epidermal_and_anatomical_studies_of_Pinus_roxburghii_needles_at_different_altitudes_in_the_North -West_Indian_Himalayas.
TYUKAVINA, O., NEVEROV, N. y KLEVTSOV, D., 2019. Influence of growing conditions on morphological and anatomical characteristics of pine needles in the northern taiga. Journal of Forest Science [en línea], vol. 65, no. 1, pp. 33-39. DOI 10.17221/126/2018-JFS. Disponible en: https://www.researchgate.net/publication/330820185_Influence_of_growing_conditions_on_morphological_and_anatomical_characteristics_of_pine_needles_in_the_northern_taiga.
ZHANG, M., MENG, J.-X., ZHANG, Z.-J., ZHU, S.-L. y LI, Y., 2017. Genetic Analysis of Needle Morphological and Anatomical Traits among Nature Populations of Pinus Tabuliformis. Journal of Plant Studies [en línea], vol. 6, no. 1, pp. 62. DOI 10.5539/jps.v6n1p62. Disponible en: https://www.researchgate.net/publication/312658131_Genetic_Analysis_of_Needle_Morphological_and_Anatomical_Traits_among_Nature_Populations_of_Pinus_Tabuliformis.
Conflict of interests:
The authors declare not to have any interest conflicts.
Authors' contribution:
The authors have participated in the writing of the work and analysis of the documents
![]()
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0
International license.
Copyright (c) 2022
Gretel Geada López, Luitmila Pérez-del Valle, Rogelio Sotolongo-Sospedra