
Cuban Journal of Forest Sciences. 2021; January-Arpril 9(1): 53-71
Translated from the original in spanish
Effect of forest successional stage on the host-epiphyte orchid relationship in the Pindo Mirador biological station, Ecuador
Efecto del estadio sucesional del bosque sobre la relación hospederos-orquídeas epífitas en la estación biológica Pindo Mirador, Ecuador
Efeito da fase sucessória da floresta sobre a relação hospedeiros- orquídeas epífitas na Estação Biológica de Pindo Mirador, Equador
Christopher Oswaldo Paredes Ulloa1* ![]() https://orcid.org/0000-0002-2087-5694
https://orcid.org/0000-0002-2087-5694
Jorge Ferro-Díaz2 ![]() https://orcid.org/0000-0001-8101-7442
https://orcid.org/0000-0001-8101-7442
Pablo Lozano Carpio3 ![]() https://orcid.org/0000-0003-0857-8141
https://orcid.org/0000-0003-0857-8141
1Provincial Decentralized Autonomous Government of Pastaza (GADPPZ). Ecuador.
2ECOVIDA Environmental Research and Services Center, Research Department, Ministry of Science, Technology and Environment. Pinar del Río, Cuba.
3Amazon State University, Research and Postgraduate Center for Amazon Conservation CIPCA, Herbarium ECUAMZ, Puyo, Pastaza. Ecuador.
*Correspondence author: chris9enginer@gmail.com
Received:08/11/2020.
Approved:12/01/2021.
ABSTRACT
Epiphyte diversity in forest ecosystems is closely related to forest structure; however, in the Pindo Mirador Biological Station in Ecuador, there are no studies documenting this interaction. Eight plots of 0.1 ha each were established, four in the secondary forest and four in the primary forest, with the objective of evaluating the effect of the current successional stage of the forest on the host-epiphyte orchid relationship in that station, based on the behavior of forest structure factors and orchid diversity. All epiphytic orchids present and host trees were recorded by measuring their diameters and heights (total and stem), comparing richness and abundance by stage and host, and applying a Canonical Correspondence Analysis between forest structure variables and epiphyte records by plots. It was detected that the main feature of the horizontal structure of orchid epiphytism is the higher abundance and lower richness in the secondary forest, occurring the opposite in the primary forest. With respect to the vertical structure, a greater abundance of orchids in the highest part of the stem of the hosts in the secondary forest, while in the primary forest they predominate at the base of the main branches, concluding that the successional stage of the forest affects both the richness and abundance of orchids on the basis of the changes experienced by the forest structure, in each of them.
Keywords: Stem height; Canopy opening; Primary forest; Secondary forest; Diameter classes.
RESUMEN
La diversidad de epífitas en los ecosistemas forestales está estrechamente relacionada con la estructura del bosque; sin embargo, en la Estación Biológica Pindo Mirador, en Ecuador, no existen estudios que documenten esa interacción. Se esTablecieron ocho parcelas de 0,1 ha cada una, estando cuatro en el bosque secundario y otras cuatro en el primario con el objetivo de evaluar el efecto del estadio sucesional actual del bosque sobre la relación hospederos - orquídeas epífitas en dicha estación, a partir del comportamiento de factores de la estructura forestal y la diversidad de orquídeas. Todas las orquídeas epífitas presentes y los árboles hospederos fueron registradas a partir de la medición de sus diámetros y alturas (total y del fuste), de comparaciones de la riqueza y abundancia por estadio y por hospederos, además de aplicarse un Análisis de Correspondencia Canónico entre variables de la estructura del bosque y los registros de epífitas por parcelas. Se detectó que el principal rasgo de la estructura horizontal del epifitismo orquideológico es la mayor abundancia y menor riqueza en el bosque secundario, ocurriendo lo contrario en el primario. Con respecto a la estructura vertical, una mayor abundancia de orquídeas en la parte más alta del fuste de los hospederos en el bosque secundario, mientras que en el primario predominan en la base de las ramas principales, concluyéndose que el estadio sucesional del bosque afecta tanto a la riqueza como a la abundancia de orquídeas sobre la base de los cambios que experimenta la estructura forestal, en cada uno de ellos.
Palabras clave: Altura de fuste; Apertura del dosel; Bosque primario; Bosque secundario; Clases diamétricas.
RESUMO
A diversidade epífita nos ecossistemas florestais está intimamente relacionada com a estrutura florestal; contudo, na Estação Biológica de Pindo Mirador, Equador, não existem estudos que documentem esta interação. Foram estabelecidas oito parcelas de 0,1 ha cada, quatro em floresta secundária e quatro em floresta primária, a fim de avaliar o efeito da sucessão atual da floresta sobre a relação orquídea-hóspede naquela estação, com base no comportamento dos fatores de estrutura florestal e diversidade de orquídeas. Todas as orquídeas epífitas presentes e as árvores hospedeiras foram registadas a partir da medição dos seus diâmetros e alturas (total e caule), comparações de riqueza e abundância por fase e por hospedeiro, e foi aplicada uma Análise de Correspondência Canónica entre as variáveis da estrutura florestal e os registos epífitos por parcela. Foi constatado que a principal característica da estrutura horizontal do epífito das orquídeas é a maior abundância e menor riqueza na floresta secundária, ocorrendo o oposto na floresta primária. No que respeita à estrutura vertical, uma maior abundância de orquídeas na parte mais alta do caule dos hospedeiros na floresta secundária, enquanto na floresta primária predominam na base dos ramos principais, concluindo que a fase sucessional da floresta afecta tanto a riqueza como a abundância de orquídeas com base nas mudanças experimentadas pela estrutura da floresta, em cada uma delas.
Palavras chave: Altura do eixo; Abertura do dossel; Floresta primária; Floresta secundária; Classes de diâmetros.
INTRODUCTION
Tropical forests are among the most biodiverse ecosystems on the planet, harboring various life forms; this high diversity results from the soil types, climate and biogeographic conditions in which they are found (Mudappa y Raman, 2007). Tropical forests are undergoing recurrent structural alterations due to global environmental changes (Stephenson et al., 2011).
Epiphytism constitutes one of the components that stand out most in tropical forests, which favors the enrichment of their biodiversity, making possible an occupation of the different strata and thus a wide range of environments for the maintenance of life that do not depend directly on the soil (Benzing, 1995; Leitman et al., 2015), making tropical forests one of the most complex ecosystems in the biosphere (Besi et al., 2019).
Understanding which environmental factors affect the functional responses of epiphytes would help to establish appropriate management and conservation strategies for these forest ecosystems, and in particular for their epiphytic component (Ferro and Delgado, 2013). However, there are few studies that enable activities for the adequate management of epiphytes in their respective ecosystems (Ferro, 2004; Hietz et al., 2006), being recurrent the fact that little is known about the impact of deforestation on the diversity of epiphytes in forests, and much less about the ecology of epiphytes in secondary forests (Barthlott et al., 2001), which is important in particular, because orchids are ecological indicators of the increase in temperatures and aridity associated with deforestation (Orta-Pozo, 2015).
The plant family of Ecuador with the greatest documented diversity is Orchidaceae, which in 2014 had 4 300 species, meaning that almost one out of every four plant species growing in the country's wild habitats is an orchid, representing more than 18% of the total number of orchid species in the world (García et al., 2014).
This family contributes the largest number of species to the phytoendemism of the country (1 707 species), approximately one third of the endemic plants are orchids (Endara et al., 2017), however, the Figures regarding the number of epiphytic orchids and their distribution by ecosystems are not precise, due to insufficient dissemination of reports that address this aspect, those that occupy mostly forest ecosystems (Bravo, 2014).
In such a scenario of the state of knowledge of the diversity of epiphytic orchids in Ecuador, studies that not only update the information on this diversity, but also provide new arguments on the interactions with the hosts, are important, which is why the studies in the Pindo Mirador Biological Station in the province of Pastaza, where there is little research on epiphytes, are of great relevance to strengthen the protection actions in the area; In this area, only a list of eight orchid species collected in its forest ecosystem has been reported for the promotion of an orchidarium of native species in the station itself (Luzuriaga et al., 2017).
Given the poor knowledge of the diversity of epiphytic orchids in the forest of the Pindo Mirador Biological Station, and mainly, on the characteristics that typify the ecology of these, both in areas with forest without anthropogenic alterations, and in the recovery dynamics that the forest is experiencing in areas with secondary forest recovered from impacts of intense logging. The study aims to assess the effect of the successional state of the forest on the host-epiphyte orchid relationship based on the behavior of forest structure factors and the distribution of the richness and abundance of epiphytic orchids in the evergreen mountain base forest in two successional stages of the Pindo Mirador Biological Station, Ecuador.
MATERIALS AND METHODS
Study area. The Pindo Mirador Biological Station (EBPM) has an area of 288.49 ha, located in the Mera canton of the Pastaza province, Ecuador, between 1100 - 1300 m a.s.l., It belongs to the Pastaza River basin and the microbasin of the Pindo Grande River and its tributary, the Plata River (Sucoshañay, 2016); the climate is mesothermal perhumid (Thornthwaite 1949), with average annual rainfall of 4 500 mm, average temperatures of 20ºC - 25ºC and average relative humidity of 88 % (Luzuriaga, 2014).
The Biological Station is located in the northern piedmont evergreen forest of the Eastern Cordillera of the Andes, in which two main zones are recognized, the Permanent Protection Zone that corresponds to the primary forest, and the Native Forest with the secondary forest, according to Luzuriaga (2014) (Figure 1).
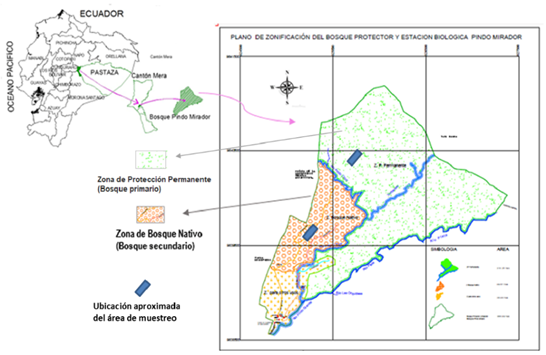
Figure 1. - Location of sampling areas
Sampling design. Eight plots of 0.1 ha each were established, covering a sampling area of 0.8 ha, four in the primary forest and four in the secondary forest, which were distributed in each successional stage. The following nomenclature was used to refer to the plots in the analyses: BP1, BP2, BP3 and BP4 are the four plots of the primary forest, while BS1, BS2, BS3 and BS4 represent the four plots of the secondary forest.
Inventory of hosts and orchids. All orchid host trees with diameters ≥ 10 cm of D1.30 were inventoried according to the methodology of Araújo et al., (2010). To those tree were measured diameter (D1.30) using a diametric tape, and the total height with a Suunto clinometer or by access to the top with a 50 m tape measure, and the height of the trunk before the first branches. The bark type of each individual tree was determined assuming two types: smooth bark and rough bark (Migenis y Ackerman 1993).
By direct visual exploration or through binoculars in each tree, epiphytic orchids (holoepiphytes according to Kress, 1986) were identified, which were determined by observation, both in the trunk of the hosts and in their branches; nomenclatural adjustments were made according to The Plant List (https://www.theplantlist.org/); the precision of endemism and recognized threat categories were obtained from Endara et al., (2017).
The abundances of orchids in each host were recorded by their life zones (phorophytic), according to their definition by Johansson (1974), modified for the first two zones of the tree trunk, given the variability of height observed, as assumed by Ferro (2004); these were: Zone 1 (Z1): from the base of the trunk to the middle of the trunk, always above 1. 30 m from the ground; Zone 2 (Z2): the rest of the trunk from the middle section of its height to the origin of the first branches; Zone 3 (Z3): the first third (basal) of the length of the main branches; Zone 4 (Z4): the middle third of the length of the branches; and Zone 5 (Z5): the last third or outside of the length of the branches.
In the interest of evaluating possible relationships between host dimensions and the presence and distribution of epiphytic orchids, tree diameter records were grouped into four classes: class 1 (CD1): 10.0 to 19.9 cm, class 2 (CD2): 20.0 to 29.9 cm, class 3 (CD3): 30.0 to 39.9 cm and class 4 (CD4): ≥ 40.0 cm; for the same purpose, tree heights were grouped into four ranges, namely: range 1 (RH1): 2.00 to 7.99 m; range 2 (RH2): 8.00 to 13.99 m; range 3 (RH3): 14.00 to 19.99 m and range 4 (RH4): 20.00 to 25.99 m; these heights were averaged by plots to establish a mean of the tree stratum in each sampling unit.
Determination of canopy cover. Hemispheric photographs were taken with a fisheye lens, using a Cannon EOS 700D camera, following the protocol proposed by Kalácska et al., (2005), with adjustments for the intermediate phase of the succession they describe, reducing the number of photographs per plot to 14, given the difference in the type of ecosystem. Using GLA (Gap Light Analyzer) vers. 2.0 software (Frazer et al., 1999), the hemispheric photographs were processed to obtain the percentage of canopy openness in each plot and to incorporate the data on this variable into the analyses of its possible effects on the distribution of epiphytic orchids.
Data analysis. Using the program PAST vers. 3.01 (Hammer et al., 2001), all descriptive statistics and their corresponding graphical representations were obtained, making a comparison of means of the richness, abundance and number of hosts between the two successional stages, using a Kruskal-Wallis test for p0.05, the same test that was used to compare the percentages of canopy openness between the two successional stages defined; the diversity per plot was also calculated according to the reciprocal Simpson's index (1/D), obtaining a comparative criterion per successional stage.
A Canonical Correspondence Analysis (CCA) was performed as suggested by Hill y Gauch (1980) to correlate a matrix of 10 environmental variables (predictors) with the abundance per plot, of the species that make up the epiphytic orchid community, and thereby determine how the orchids adjust according to their relationship with the effects produced by a set of variables of the forest ecosystem; for this purpose the software MVSP vers. 3.22-demo- (Kovach Computing Services, 1985-2013: https://www.kovcomp.co.uk/downl2.html); the environmental variables were: number of host trees per each of the four established diameter classes (criterion of preference or not for host thickness), abundance of shafts in the forest (criterion of influence of tree density), average canopy openness per plot (criterion of preference or not for more or less illuminated environments), average height of the trees in the forest (criterion of the effect of the height of the plant formation stratification), average height of the trunk (criterion of the effect of the trunk support), and the two types of bark (smooth and rough) of the host shafts (criterion of preference for epiphyte fixation), and the two types of bark (smooth and rough) of the host trunk (criterion of preference for epiphyte fixation).
RESULTS AND DISCUSSION
Richness and abundance of epiphytic orchids; effect of successional stage
Forty-nine species of epiphytic orchids were identified (both successional stages), 35 species in the secondary forest (BS), 48 in the primary forest (BP); 14 species that were located in the BP were not observed in the BS sampling, as well as a single undetermined species of the genus Epidendrum present only in the BS. Such richness belongs to 20 genera, with Maxillariella (10 species) followed by Elleanthus (8 species) and Epidendrum (6 species) being the best represented. Of the total, six species are recognized as endemic to Ecuador: Dichaea sodiroi Schltr, Epidendrum puyoense Hágsater y Dodson, Pleurothallis alveolata Luer, Sievekingia hirtzii Waldvogel, Sievekingia marsupialis Dodson and Stelis anolis Luer, which in turn have Threatened category: 4 Near Threatened -NT- and 2 Vulnerable -V- according to Endara et al., (2017).
The total abundance of epiphytes is 2158 individuals, with a higher Figure in the BS, where 1248 were counted, while in the BP the Figure was 910. The differentiation of species richness and abundance by successional stage is shown in Figure 2 (A and B), which also reflects the greater range of variation in the BS records for both species richness and abundance.
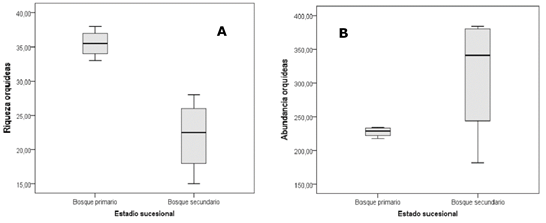
Figure 2. - Variation in richness (A) and abundance (B) of epiphytic orchids by successional stage
The results found for the secondary forest are similar to those described by Eizmann y Zotz (2016) in patches of secondary forest in Panama, which in their interpretation coincide with the effect of rapid expansion of epiphytes in the early stage of their post-disturbance dynamics. A greater abundance of orchids is observed in the BS, where hosts are also more abundant; in the case of the BP, the most important feature is the greater richness of epiphytic orchid species.
A similar feature had been found by Barttlot et al., (2001) and Ferro (2004), who state that, in a recuperative dynamic, when the forest ecosystem is in a more estable and mature state, the richness of epiphytes is greater than in the stages closer to the disturbance, as well as their abundances are regulated with the successional transit, something that is ratified by this study.
Distribution of host trees and richness and abundance of epiphytic orchids by successional stages.
Table 1 shows the behavior of the sampling in each plot, and also verifies that these differences are maintained in relation to the number of host trees, such a distribution of richness and abundance.
Table 1. - Richness of epiphytic orchids (S), their abundance and number of trees hosting these epiphytes by successional stage

The reported records of richness and abundance of epiphytic orchids in all sampling plots (Table 1), offers a clear differentiation of each of the variables between BP and BS, verifying that the BP is characterized by a greater richness of species, which on average resulted in 35.5 species for the BP and 22 for the BS, a difference that is statistically significant (p=0.021); The variation in the abundance of orchids between the two successional stages was also observed, being higher in the BS than in the BP, a numerical difference that was not statistically significant (p=0.021). (p=0,248).
From these results it can be interpreted that there is an effect of the successional stage for species richness and abundance, being this effect more marked on the variation of richness, which increases as the forest is more mature, however when it is recovering from relatively recent impacts of deforestation, the orchid community becomes more established, probably from the flow coming from the surrounding matrix of primary forest, but with a more aggressive distribution of its abundance, derived from the conditions that the forest reaches at this stage in its potential host network, a statement that is supported by the fact observed in Table 1, that in the BS a lower species richness occupies more trees to sustain itself, a reason that influences the increase in its abundance with respect to the BP.
Species richness agrees with that reported by authors such as Barthlott et al. (2001), who found in secondary forests of the Venezuelan Andes 36 species of epiphytic orchids in an area of 0.3 ha; for their part Einzmann y Zotz (2016) found 24 species in seven patches of secondary forest in Panama; they also conform to the reports of Hietz et al., (2006) and Besi et al., (2019). Guzmán-Jacob et al., (2020) demonstrate that nearby mature forest sources are important in epiphyte abundance, all of which fits the situation observed in Pindo Mirador; also supporting the criteria set forth in results such as those of Mongosongo y Griffiths (2019), who argue that both epiphyte richness and abundance increase in more mature forests due to their greater host capacity of phorophytes, which preserves the variations required for greater numbers of species.
Given the described behavior of the richness and abundance in each sample for both successional stages, a greater diversity is verified in the primary forest with respect to the secondary forest (Figure 3), according to the values of the calculated reciprocal Simpson index (1/D), which presents values always higher than 0, 92 in BP confirming a higher probability in the occurrence of individuals of the same species due to the fact that the number of these is higher in the community, something that is lower in the secondary forest (Simpson1/D ≤ 0.87), even with its higher abundances, due to the significant difference in its species richness with respect to the primary forest.
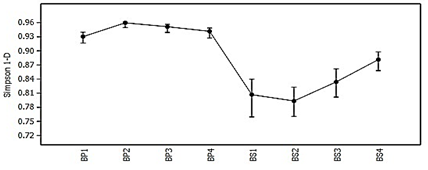
Figure 3. - Behavior of diversity according to Simpson's Index (1-D) at each successional stage
Relationship between trees and orchids by successional stage
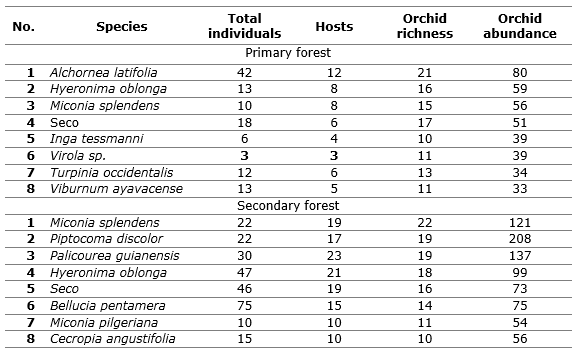
With respect to tree richness and its contribution to epiphytism, as shown in Table 2, it was observed that it is typified by a higher frequency of epiphytic orchids in seven hosts (six species plus dry trees), of which are in the primary forest Alchornea latifolia Swart, Hyeronima oblonga (Tul.) Müll. Arg., Miconia splendens (Sw.) Griseb, Inga tessmanni Harms, Virola sp., Turpinia occidentalis (Sw.) G. Don and Viburnum ayavacense Kunth, with 52 containing orchids, of the 117 individuals observed; in such diversity of hosts 391 individuals of epiphytic orchids were found and an average richness of 14 orchid species per tree.
Table 2 shows that in the case of secondary forest, the hosts with the highest number of orchid species were Miconia splendens (Sw.) Griseb, Piptocoma discolor (Kunth) Pruski, Palicourea guianensis Aubl, Hyeronima oblonga (Tul.) Müll. Arg., Bellucia pentamera Naudin. plus dry trees, which in total grouped 267 individuals, and 174 of them were hosts. In these secondary forest host trees, an abundance of 823 epiphytic orchids and an average richness of 16 species per tree were observed.
Table 2. -Trees with the greatest contribution to the richness and abundance of epiphytic orchids in the two successional stages evaluated
Distribution of epiphytic orchids by diameter classes and phorophytic zones; effect of successional stage
The distribution of epiphytic orchids in relation to the diameter classes of their hosts (Figure 4 A and B) shows the variation established in each successional stage, observing in the BP a greater presence in trees with stem diameters between 20 and 40 cm, a characteristic that fits the dominance in this successional stage of trees of larger dimensions than those of the BS, as exposed by Luzuriaga et al., (2011), since the latter stage is dominated by those between 10 and 30 cm D1.30, which coincides with studies documenting increases in diameter dimensions with the advancement of successional dynamics, and reflects that mature stages offer greater capacities in host architecture to host epiphytism, which is exposed by Adhikari et al., (2017) and Hernández-Pérez et al., (2018).
The vertical distribution of epiphytic orchids according to their location in each of the five forophytic zones (Figure 4 C and D) confirms the effect of the successional stage, which influences the dominant position in the upper section of the trunks (in secondary forest), or at the base of the main branches of the canopy (in primary forest).

Figure 4. - Distribution of epiphytic orchids according to diameter classes (A and B) and
phorophytic zones (C and D)
in the two successional stages evaluated (primary forest and secondary forest)
Canopy openness
The analysis of the percentage of canopy openness shows that in the BP the canopy is more closed than in the BS (Figure 5), which is statistically significant (p=0.0209); This suggests that the effect of successional stage on the vertical distribution of orchid epiphytism in Pindo Mirador is related to the structure of the forest canopy, causing orchids to concentrate more in a zone of canopy protection such as Z3; however, as the canopy is more open in BS, epiphytes descend, taking advantage of the greater protection from solar radiation penetration. Such a perspective supports a similar pattern obtained by Hernández-Pérez et al., (2018), and also supported by the arguments of Johansson (1974) about the effect of environmental conditions under the canopy according to the time in which the forest structure changes in its successional dynamics.

Figure 5. - Distribution of the percentage of canopy openness in the two successional
stages
(primary forest-Bprim- and secondary forest-Bsec- and secondary forest-Bsec-)
Correspondence between forest structure variables and plots, according to the richness and abundance of epiphytic orchids observed
The forest structural variables (predictors) assumed to be correlated with epiphytic orchid sampling (sampling sites) at each successional stage (Table 3) represent a measure of how changes in forest structure at both successional stages relate to variation in orchid epiphytism show changes from one stage to the others (Table 3), among which are recognized as the clearest tree heights, including their shafts, percent canopy openness and the two types of bark of the assumed trees.
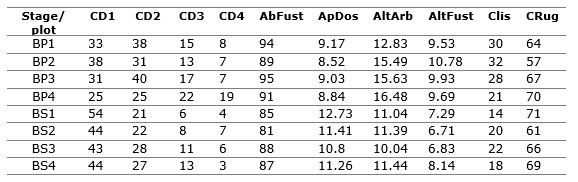
Tabla 3. - Values of forest structure variables assumed in the ACC by plots and by successional stage, where CD1 is Diameter Class 1, CD2 is Diameter Class 2, CD3 is Diameter Class 3, CD4 is Diameter Class 4, AbFust is canopy abundance, ApDos is canopy openness in percent, AltArb is tree height (average), AltFust is canopy height (average), Clis is smooth bark and Crug is rough bark
The canonical correspondence analysis (CCA) shows that the first two axes show a relatively good solution to the ordination of species and plots according to the 10 environmental variables, which explain 58.78 % of the total variability (Table 4), which including a third axis represents the accumulated 72.09 % of the total variability (Table 4).
Table 4. - Results of the Canonical Correspondence Analysis (CCA) of the eight plots by successional stages according to the environmental variables

The biplot graph (Figure 6) shows the projection of environmental variables and sampling plots by successional stage. Two groups can be seen, one related to the secondary forest, where the dispersion of the sampling is observed, with little effect of variables; influence of those more linked to a dynamic close to the impacts of logging, such as the predominance of trees of smaller diametric size (CD1) and a higher percentage in the opening of the canopy (ApDos).
In the primary forest there is a greater effect of variables related to a mature forest structure (greater height of the trees and their trunks, the abundance of trees and the greater diametric dimensions of their trunks).
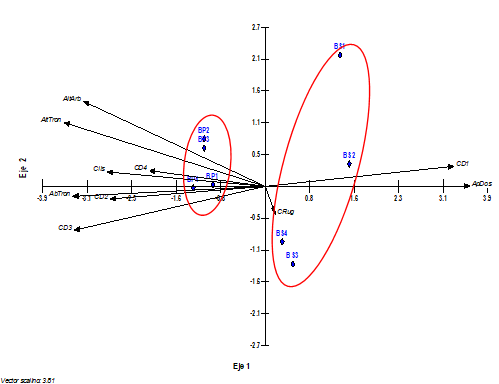
Figure 6. - Projection of environmental variables and epiphytic orchid sampling plots according to the ACC. The environmental variables are shown as arrows while the plots are shown as blue triangles, where BP and BS correspond to the successional stage and the accompanying number to the number of sampling plots
The results of the above analysis are similar to the work of Krömer and Gradstein, (2003); Hietz et al., (2006); Flores-Palacios (2011) and García-Franco, 2008 y Larrea and Werner (2010) that agree that environmental changes caused by the elimination or modification of the structure of the natural forest cause changes in the composition, relative abundance, spatial distribution and functional responses of epiphytes.
The results show that the successional stage represents a structure that varies according to whether or not the proximity of intense logging impacts persists in the forest. Benzing (1995) and Köster et al., (2009) argued that successional changes in forest ecosystems affect their epiphyte communities, mainly by variations in the structure of the canopy that influences the light regime, humidity and temperature under it; a fact that supports the differentiation observed in Pindo Mirador, and thus confirms an effect of the successional stage on such environmental conditions within the forest, and consequently in the characterization of its orchid epiphytism.
CONCLUSIONS
The successional state of the forest affects both the richness and abundance of epiphytic orchids, with greater relevance in their vertical distribution, being higher the contribution of variations in the height of the main stems and branches of the trees.
The main structural characteristics of the orchid community are typified by a higher abundance and lower richness of species in the BS than in the BP, located in their hosts according to the dominant trees and mostly in the upper part of the trunks in the BS, and at the base of the main branches in the BP.
REFERENCES
ADHIKARI, Y.P., FISCHER, A., FISCHER, H.S., ROKAYA, M.B., BHATTARAI, P. y GRUPPE, A., 2017. Diversity, composition and host-species relationships of epiphytic orchids and ferns in two forests in Nepal. Journal of Mountain Science [en línea], vol. 14, no. 6, pp. 1065-1075. [Consulta: 14 de mayo de 2020]. ISSN 1993-0321. DOI 10.1007/s11629-016-4194-x. Disponible en: https://doi.org/10.1007/s11629-016-4194-x.
BARTHLOTT, W., SCHMIT-NEUERBURG, V., NIEDER, J. y ENGWALD, S., 2001. Diversity and abundance of vascular epiphytes: a comparison of secondary vegetation and primary montane rain forest in the Venezuelan Andes. Plant Ecology [en línea], vol. 152, no. 2, pp. 145-156. [Consulta: 14 de mayo de 2020]. ISSN 1573-5052. DOI 10.1023/A:1011483901452. Disponible en: https://doi.org/10.1023/A:1011483901452.
BENZING, D.H., 1995. THE PHYSICAL MOSAIC AND PLANT VARIETY IN FOREST CANOPIES. Selbyana [en línea], vol. 16, no. 2, pp. 159-168. [Consulta: 14 de mayo de 2020]. ISSN 0361-185X. Disponible en: https://www.jstor.org/stable/41759902.
BRAVO, E., 2014. La Biodiversidad en el Ecuador [en línea]. Ecuador: Editorial Universitaria Abya-Yala. [Consulta: 16 julio 2020]. Disponible en: http://190.57.147.202:90/jspui/bitstream /123456789/303/1/La%20Biodiversidad.pdf.
EINZMANN, H.J.R. y ZOTZ, G., 2016. How Diverse are Epiphyte Assemblages in Plantations and Secondary Forests in Tropical Lowlands? Tropical Conservation Science [en línea], vol. 9, no. 2, pp. 629-647. [Consulta: 17 julio 2020]. ISSN 1940-0829. DOI 10.1177/194008291600900205. Disponible en: https://doi.org/10.1177/194008291600900205.
ENDARA, L., HIRTZ, A. y JOST, L., 2017. Libro Rojo de Plantas Endémicas del Ecuador. Quito, Ecuador: Publicaciones del Herbario QCA, Pontificia Universidad Católica del Ecuador.
ENTALAI, E., NIKONG, D., MUSTAFA, M. y GO, R., 2019. Orchid diversity in antropogenic-induced degraded tropical rainforest, an extrapolation towards conservation. Lankesteriana [en línea], vol. 19, no. 2. DOI 10.15517/lank.v19i2.38775. Disponible en: https://www.researchgate.net/publication/335434623_Orchid_diversity_in_antropogenic-induced_degraded_tropical_rainforest_an_extrapolation_towards_conservation.
FERRO DÍAZ, J., 2004. Efecto del aprovechamiento forestal sobre la estructura y dinámica de la comunidad de epifitas vasculares del bosque semideciduo notófilo de la Península de Guanahacabibes. Tesis en opción al grado científico Doctor en Ciencias Forestales. Pinar del Río, Cuba: Universidad de Pinar del Río «Hermanos Saíz Montes de Oca».
FERRO, J. y DELGADO, F., 2013. Dinámica post disturbio de claros del dosel en el bosque tropical seco semideciduo de la Península de Guanahacabibes, Cuba; su relación con la abundancia de epífitas vasculares. En: L. FERNÁNDEZ y A. VOLPEDO (eds.), Evaluación de los cambios de estado de ecosistemas degradados de Iberoamérica [en línea]. Buenos Aires, Argentina: Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo CYTED, pp. 200-213. Disponible en: https://es.scribd.com/document/327975559/Evaluacion-de-Los-Cambios-de-Estado-en-Ecosistemas-Degradados-de-Iberoamerica.
FLORES-PALACIOS, A., GARCÍA-FRANCO, J.G., VALENCIA-DÍAZ, S., SOLÍS-MONTERO, L. y CRUZ-ANGÓN, A., 2011. Diversidad y conservación de plantas epífitas vasculares en el centro del Estado. En: COMISIÓN NACIONAL PARA EL CONOCIMIENTO Y USO DE LA BIODIVERSIDAD (ed.), La Biodiversidad en Veracruz: Estudio de Estado [en línea]. México: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Universidad Veracruzana : Instituto de Ecología, pp. 493-501. ISBN 978-607-7607-50-2. Disponible en: http://bibliotecasibe.ecosur.mx/sibe/book/000051158.
FRAZER, G.W., CANHAM, C.D. y LERTZMAN, K.P., 1999. Gap Light Analyzer (GLA): Imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, user’s manual and program documentation. [en línea]. Millbrook, New York: University, Burnaby, British Columbia, and the Institute of Ecosystem Studies. Disponible en: http://rem-main.rem.sfu.ca/downloads/Forestry/GLAV2UsersManual.pdf.
GARCÍA, M., PARRA, D. y MENA, P., 2014. El país de la biodiversidad: Ecuador. Fundación Botánica de los Andes. Quito, Ecuador: Ministerio del Ambiente y Fundación EcoFondo.
GARCÍA-FRANCO, J.G. y TOLEDO, T., 2008. Epífitas vasculares: bromelias y orquídeas. En: R. MANSON, S. HERNÁNDEZ-ORTIZ y K. MEHLTRETER (eds.), Agroecosistemas cafetaleros de Veracruz. Biodiversidad manejo y conservación [en línea]. México: Instituto de Ecología/Instituto Nacional de Ecología, pp. 69-82. Disponible en: http://www2.inecc.gob.mx/publicaciones2/libros/542/cap5.pdf.
GUZMÁN‐JACOB, V., ZOTZ, G., CRAVEN, D., TAYLOR, A., KRÖMER, T., MONGE‐GONZÁLEZ, M.L. y KREFT, H., 2020. Effects of forest-use intensity on vascular epiphyte diversity along an elevational gradient. Diversity and Distributions [en línea], vol. 26, no. 1, pp. 4-15. [Consulta: 8 marzo 2021]. ISSN 1472-4642. DOI https://doi.org/10.1111/ddi.12992. Disponible en: https://onlinelibrary.wiley.com/doi/abs/10.1111/ddi.12992.
HAMMER, O., HARPER, D. y RYAN, P., 2001. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica [en línea], vol. 4, pp. 1-9. Disponible en: https://www.researchgate.net/publication/259640226_PAST_Paleontological_Statistics_Software_Package_for_Education_and_Data_Analysis.
HERNÁNDEZ-PÉREZ, E., SOLANO, E., RÍOS-GÓMEZ, R., HERNÁNDEZ-PÉREZ, E., SOLANO, E. y RÍOS-GÓMEZ, R., 2018. Host affinity and vertical distribution of epiphytic orchids in a montane cloud forest in southern Mexico. Botanical Sciences [en línea], vol. 96, no. 2, pp. 200-217. [Consulta: 6 de agosto de 2020]. ISSN 2007-4298. DOI 10.17129/botsci.1869. Disponible en: http://www.scielo.org.mx/scielo.php?script=sci_abstract&pid=S2007-42982018000200200&lng=es&nrm=iso&tlng=en.
HIETZ, P., BUCHBERGER, G. y WINKLER, M., 2006. Effect of forest disturbance on abundance and distribution of epiphytic bromeliads and orchids. Ecotropica [en línea], vol. 12, pp. 103-112. Disponible en: https://www.researchgate.net/publication/236685338_Effect_of_forest_disturbance_on_abundance_and_distribution_of_epiphytic_bromeliads_and_orchids.
HILL, M.O. y GAUCH, H.G., 1980. Detrended correspondence analysis: An improved ordination technique. Vegetatio [en línea], vol. 42, no. 1, pp. 47-58. [Consulta: 18 de junio de 2020]. ISSN 1573-5052. DOI 10.1007/BF00048870. Disponible en: https://doi.org/10.1007/BF00048870.
JOHANSSON, D., 1974. Ecology of vascular epiphytes in West African rain forest [en línea]. S.l.: Acta Phytogeographica Suecica. [Consulta: 16 julio 2020]. Disponible en: http://www.diva-portal.org/smash/get/diva2:565496/FULLTEXT01.pdf.
KALÁCSKA, M., CALVO-ALVARADO, J.C. y SÁNCHEZ-AZOFEIFA, G.A., 2005. Calibration and assessment of seasonal changes in leaf area index of a tropical dry forest in different stages of succession. Tree Physiology [en línea], vol. 25, no. 6, pp. 733-744. ISSN 0829-318X. DOI 10.1093/treephys/25.6.733. Disponible en: https://pubmed.ncbi.nlm.nih.gov/15805093/.
KÖSTER N, et al., 2009. Conservation of epiphyte diversity in an Andean landscape transformed by human land use. Conservation Biology : the Journal of the Society for Conservation Biology [en línea], vol. 23, no. 4, pp. 911-919. [Consulta: 18 de junio de 2020]. ISSN 0888-8892, 1523-1739. DOI 10.1111/j.1523-1739.2008.01164.x. Disponible en: https://europepmc.org/article/med/19210304.
KRÖMER, T. y GRADSTEIN, S.R., 2003. Species Richness of Vascular Epiphytes in Two Primary Forests and Fallows in the Bolivian Andes. Selbyana [en línea], vol. 24, no. 2, pp. 190-195. [Consulta: 18 de junio de 2020]. ISSN 0361-185X. Disponible en: https://www.jstor.org/stable/41760132.
LARREA, M.L. y WERNER, F.A., 2010. Response of vascular epiphyte diversity to different land-use intensities in a neotropical montane wet forest. Forest Ecology and Management [en línea], vol. 260, no. 11, pp. 1950-1955. [Consulta: 8 marzo 2021]. ISSN 0378-1127. DOI 10.1016/j.foreco.2010.08.029. Disponible en: https://www.sciencedirect.com/science/article/pii/S0378112710004883
LEITMAN, P., AMORIM, A.M., SANSEVERO, J.B.B. y FORZZA, R.C., 2015. Floristic patterns of epiphytes in the Brazilian Atlantic Forest, a biodiversity hotspot. Botanical Journal of the Linnean Society [en línea], vol. 179, no. 4, pp. 587-601. [Consulta: 18 de junio de 2020]. ISSN 1095-8339. DOI https://doi.org/10.1111/boj.12342. Disponible en: https://onlinelibrary.wiley.com/doi/abs/10.1111/boj.12342.
LUZURIAGA, C., CUASAPAZ, C. y QUICHIMBO, G., 2011. INVENTARIO FORESTAL EN LA ESTACIÓN PINDO MIRADOR. Tsafiqui - Revista Científica en Ciencias Sociales [en línea], no. 2, pp. 83-107. [Consulta: 18 de junio de 2020]. ISSN 2602-8069. DOI 10.29019/tsafiqui.v0i2.211. Disponible en: https://revistas.ute.edu.ec/index.php/tsafiqui/article/view/211.
LUZURIAGA, C.X., QUICHIMBO, G.I. y BLANCO, J., 2017. Viverización de orquídeas epífitas como estrategia de conservación de especies autóctonas en los bosques amazónicos de Pastaza (Ecuador). Memorias del 7mo. Congreso Forestal Español [en línea]. Ecuador: Sociedad Española de Ciencias Forestales, Disponible en: http://7cfe.congresoforestal.es/content/viverizacion-de-orquideas-epifitas-como-estrategia-de-conservacion-de-especies-autoctonas-en.
LUZURIAGA Q, C.X., 2014. INVENTARIO FLORÍSTICO DEL BOSQUE QUE RODEA A LA LAGUNA EN LA ESTACIÓN BIOLÓGICA PINDO MIRADOR PASTAZA-ECUADOR. Tsafiqui - Revista Científica en Ciencias Sociales [en línea], no. 6, pp. 15-25. [Consulta: 18 de junio de 2020]. ISSN 2602-8069. DOI 10.29019/tsafiqui.v0i6.228. Disponible en: https://revistas.ute.edu.ec/index.php/tsafiqui/article/view/228.
MIGENIS, L.E. y ACKERMAN, J.D., 1993. Orchid—phorophyte relationships in a forest watershed in Puerto Rico. Journal of Tropical Ecology [en línea], vol. 9, no. 2, pp. 231-240. [Consulta: 8 marzo 2021]. ISSN 1469-7831, 0266-4674. DOI 10.1017/S0266467400007227. Disponible en: https://www.cambridge.org/core/journals/journal-of-tropical-ecology/article/abs/orchidphorophyte-relationships-in-a-forest-watershed-in-puerto-rico/6B4579E8A64C56E734836F38ADEEDDC9#.
MUDAPPA, D., RAMAN, T.R.S., SHAHABUDDIN, G. y RANGARAJAN, M., 2007. Rainforest restoration and wildlife conservation on private lands in the Western Ghats. Making conservation work [en línea]. India: Permanent Black, Ranikhet, pp. 210-240. Disponible en: https://www.researchgate.net/publication/258699793_Rainforest_restoration_and_wildlife_conservation_on_private_lands_in_the_Western_Ghats.
STEPHENSON, N.L., MANTGEM, P.J. van, BUNN, A.G., BRUNER, H., HARMON, M.E., O’CONNELL, K.B., URBAN, D.L. y FRANKLIN, J.F., 2011. Causes and implications of the correlation between forest productivity and tree mortality rates. Ecological Monographs [en línea], vol. 81, no. 4, pp. 527-555. [Consulta: 18 de junio de 2020]. ISSN 1557-7015. DOI https://doi.org/10.1890/10-1077.1. Disponible en: https://esajournals.onlinelibrary.wiley.com/doi/abs/10.1890/10-1077.1.
SUCOSHAÑAY, D.J., 2016. Propuesta para el ordenamiento ambiental de la Cuenca del Río Puyo, en la amazonía ecuatoriana [en línea]. Tesis doctoral. La Habana, Cuba: Facultad de Geografía, Universidad de La Habana. Disponible en: http://eduniv.reduniv.edu.cu/index.php?page=13&id=272&db=1.
THORNTHWAITE, C.W., BIEL, E.R., CHURCH, P.E., JACOBS, W.C., LANDSBERG, H., LEIGHLY, J.B. y HAFSTAD, K., 1949. Report of the Committee on Climatology, 1947–1948. Eos, Transactions American Geophysical Union [en línea], vol. 30, no. 3, pp. 439-443. [Consulta: 18 de junio de 2020]. ISSN 2324-9250. DOI https://doi.org/10.1029/TR030i003p00439. Disponible en: https://agupubs.onlinelibrary.wiley.com/doi/abs/10.1029/TR030i003p00439.
Conflict of interests:
Los autores declaran no tener conflictos de intereses.
Authors' contribution:
Christopher Oswaldo Paredes Ulloa: Conception of the idea, literature search and review, instrument making, instrument application, compilation of information resulting from the instruments applied, statistic analysis, preparation of tables, graphs and images, database preparation, drafting of the original (first version), review and final version of the article, authorship coordinator, translation of terms or information obtained, review of the application of the applied bibliographic standard.
Jorge Ferro-Díaz: Conception of the idea, literature search and review, instrument making, instrument application, compilation of information resulting from the instruments applied, statistic analysis, preparation of tables, graphs and images, database preparation, general advice on the topic addressed, review and final version of the article, article correction, translation of terms or information obtained.
Pablo Lozano Carpio: Literature search and review, instrument making, preparation of tables, graphs and images, general advice on the topic addressed, review and final version of the article, article correction.
![]()
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International license.
Copyright (c) 2021 Christopher Oswaldo Paredes Ulloa, Jorge Ferro-Díaz, Pablo Lozano Carpio