
Revista Cubana de Ciencias Forestales. 2020; September-December 8(3): 495-506
Translated from the original in spanish
Germination power of Polylepis incana with application of different water treatments
Poder germinativo de Polylepis incana con aplicación de diferentes tratamientos de agua
Poder germinativo da Polylepis incana com aplicação de diferentes tratamentos de água
Ángel Canales Gutiérrez1* ![]() https://orcid.org/0000-0002-3096-1705
https://orcid.org/0000-0002-3096-1705
Yanina Ruth Huarasa Vilca1![]() https://orcid.org/0000-0001-8700-8192
https://orcid.org/0000-0001-8700-8192
1Institución de adscripción: Programa de Ecología de la Facultad de Ciencias Biológicas de la Universidad Nacional del Altiplano de Puno. Perú
*Correspondence author: acanales@unap.edu.pe
Received: August 31th, 2020.
Approved: October 2nd, 2020.
ABSTRACT
Polylepis incana Kunth (queñoa), is a species that has diverse local uses; however, it has a low germination power in natural conditions. The objective of this research was to compare the germination power of Polylepis incana, with coconut, residual and untreated water treatments. An experiment was set up with three types of water and five doses of water (5, 10, 15, 20 and 25 ml). The germination was developed in 15 trays, in each one 750 g of substrate and 20 seeds were added. The irrigation was done daily with different amounts of water, from 5 to 25 ml. The percentage of germination power, temperature and soil pH were recorded daily. The statistical analyses were carried out in the INFOSTAT program. The highest germination power of seeds was registered with irrigation with residual water (9 %), while seeds irrigated with coconut and untreated water reached 4 %. Therefore, a 9 % germination power of P. incana seeds, irrigated with residual water, was obtained.
Keywords: Germination; Greenhouse; Polylepis incana; Irrigation; Treatment.
RESUMEN
Polylepis incana Kunth (queñoa), es una especie que posee diversos usos locales, sin embargo, presenta un bajo poder germinativo en condiciones naturales. El objetivo de la investigación fue comparar el poder germinativo de Polylepis incana, con tratamientos de agua de coco, residual y de pozo. Se instaló un experimento con tres tipos de agua y cinco dosis de agua (5, 10, 15, 20 y 25 ml). La germinación se desarrolló en 15 bandejas, en cada una se adicionó 750 g de sustrato y 20 semillas. El riego se realizó diariamente con diferentes cantidades de agua, desde 5 a 25 ml. Se registró el porcentaje de poder germinativo, temperatura y pH del suelo de forma diaria. Los análisis estadísticos fueron realizados en el programa INFOSTAT. El mayor poder germinativo de semillas, se registró con riegos con agua residual (9 %), mientras que las semillas regadas con agua de coco y de pozo alcanzaron un 4 %. Por tanto, se ha obtenido un 9 % de poder germinativo de semillas de P. incana, regadas con agua residual.
Palabras clave: Germinación; Invernadero; Polylepis incana; Riego; Tratamiento.
RESUMO
Polylepis incana Kunth (queñoa), é uma espécie que tem várias utilizações locais, no entanto, apresenta um baixo poder germinativo em condições naturais. O objetivo da investigação era comparar o poder germinativo da Polylepis incana, com tratamentos de coco, resíduos e água de poços. Foi realizada uma experiência com três tipos de água e cinco doses de água (5, 10, 15, 20 e 25 ml). A germinação foi desenvolvida em 15 tabuleiros, em cada um deles foram adicionados 750 g de substrato e 20 sementes. A irrigação foi feita diariamente com diferentes quantidades de água, de 5 a 25 ml. A percentagem do poder germinativo, temperatura e pH do solo foram registados diariamente. As análises estatísticas foram levadas a cabo no programa INFOSTAT. O maior poder germinativo das sementes foi registado com irrigação com água residual (9 %), enquanto as sementes irrigadas com coco e água de poço atingiram 4 %. Portanto, 9 % do poder germinativo foi obtido a partir de sementes de P. incana irrigadas com águas residuais.
Palavras-chave: Germinação; Estufa; Polylepis incana; Irrigação; Tratamento.
INTRODUCTION
Polylepis incana (Wesche et al., 2008), is an angiosperma, dicotyledonous (Renison and Cingolani 1998), has a great diversity of species that are present in South America (Montesinos-Tubée et al., 2015; Abdellaoui et al., 2019) and distributed along the Andes (Vega-Krstulovic et al., 2007). The greatest diversity of species is found between the altitudes of 3 000 m a.s.l. (Kessler and Schmidt-Lebuhn 2006) to 4 600 m a.s.l. (Domic and Capriles 2009), being trees and some, shrubs (Mendoza and Cano 2011).
The species P. incana, is endemic to Peru (Castro and Flores 2015), has a height of two to five meters (Seltmann et al., 2007), is characterized mainly by twisted stems (Argibay and Renison 2018) and slow growth (Domic et al., 2013), but with adaptations to low temperatures (Hidalgo et al., 2013).
Polylepis forests are at present one of the most vulnerable ecosystems in the Americas (Castro and Flores 2015), due to environmental factors (Arana-Paredes et al., 2015), low rates of regeneration (Argollo et al., 2004; Seltmann et al., 2007), intensified land use (Argollo et al., 2004), climate change (Domic et al., 2013), intense pressure for agricultural expansion (Domic et al., 2017) and habitat degradation (Seltmann et al., 2007).
Various efforts have been made to promote the germination and propagation of the P. incana species, however, there are difficulties in genetic reproductive processes, which are characteristic of the plant (Zutta et al., 2012), such as restrictions on temperature variations (Landi and Renison 2010), low natural seed dispersal (Wesche et al., 2008), this causes slow regeneration of the Polylepis bosques (Enrico et al., 2004). From the seeds collected in the natural environment, only 10 % are optimal for sowing (Montesinos-Tubée et al., 2015), they are affected by low temperature in their natural habitat (Simoes and Renison 2015; Pulido and Ramos 2016), soil evapotranspiration (Rosero et al., 2018), climate variability (Zutta et al., 2012) with unpredictable effects on seed germination.
Several studies have shown that the P. incana, has low percentage of germination and can reach only between 3 % and 5 % (Enrico et al., 2004; Domic et al., 2017), with the possibility of germinating in different substrates: sand, soil, rocks and good preparation of the soil (Renison and Cingolani 1998; Olivera et al., 2018). The good development of the seeds depends on the parameters of the quality of the water used for irrigation (Torres et al., 2008); the environmental conditions in which it is found (Renison et al., 2004) and the osmotic adjustments of the seed (Domic and Capriles 2009).
The treated water can be used to irrigate plants (Passarini et al., 2012). Likewise, irrigation with waste water enhances the germination capacity and fertility of the seedling (Cardonell et al., 2012), because it has a large amount of nutrients such as nitrogen and phosphorus (Beltrán et al., 2015). For example, coconut water promotes germination at maximum levels (Patiño et al., 2011), because it contains hormones with cytokinin action of the isoprenoid type, which promotes the process of cell division, thus influencing the post-germination process (Del Pozo et al., 2005; Quinto et al., 2009), with coconut water promoting germination, with temperatures between 16°C and 20°C (Arana et al., 2015).
The importance of investigating the germinative viability of P. incana, is based on the use made by local populations (Capriles and Flores 2002), such as: use as firewood (Kessler and Schmidt 2006; Wesche et al., 2008) and medicinal, as it acts as antihypertensive (Daud et al., 2007), and also as fodder for animals (Castañeda and Albán 2016). The protection and conservation of the forests of P. incana, from the sowing by seeds, is by the vegetal cover that provides in the Andean ecosystems (Schmidt-Lebuhn et al., 2006), by its participation in the regulation of the runoff, control of the erosion (Delgado and Leon-Vargas 2017), its capacity to retain and to capture water, being the main type of cover in the hydrographic basins high Andean(Enrico et al., 2004).
The cold damages the seeds, causing physiological damage and slowing down the growth, therefore, it is appropriate to propagate in spring; the seed-germinated plants are more feasible than the seedlings by stake (Vasco 2010), the seeds of P. incana, have a high degree of impurity (Vega et al., 2018). The germination responses of Polylepis incana seeds under greenhouse conditions show a higher germination percentage of approximately 19 %.
The objective of the investigation was to compare the germinative power of Polylepis incana, with water treatments (residual, coconut and untreated).
MATERIALS AND METHODS
Study area
The research was conducted in the greenhouse of the Environmental Management Office of the National University of the Altiplano of Puno, located at 15°49'34" south latitude, 70°00'19" west longitude and at an altitude of 3816 m a.s.l. (Figure 1).
Figure 1 - Location of the greenhouse of the Universidad Nacional del Altiplano in Puno, Perú
Design of the experiment
The sowing was done in vitro, 300 seeds of P. incana were sown, in fifteen plastic trays, 20 seeds were distributed per tray with the help of sterile dissecting forceps. They were placed inside trays of 100 g of substrate sifted with black soil, sand and sheep manure; the trays with seeds were placed on a table under the light intensity of the sun.
To evaluate the effect of the type of water, coconut water was used, having purchased 13 coconuts for the whole process of the investigation; contaminated water was extracted from the interior bay of Puno and the untreated water from a spring located in the city of Puno (Jr. Independencia and Av. La Torre). Likewise, the environmental temperature of the greenhouse was recorded where an average of 25°C was obtained.
The experiment had 5 doses of irrigation with three treatments; the first treatment (T1), was of coconut water, the first was with doses of irrigation of 5 ml, the second repetition was with 10 ml, the third was with 15 ml, the fourth was with 20 ml and the fifth was with 25 ml. With the second treatment (T2) it was of residual water and the third treatment (T3) with untreated water, the doses were with the same amounts of irrigation for the three treatments.
The pH, humidity and temperature (°C) of the substrate were monitored weekly; the pH and moisture were measured with a HANNA pH meter, Checker, USA and the temperature with an Oaklon infrared thermometer, Mini infraPro 6, USA.
Evaluation of germination
In order to evaluate the germination in vitro, the number of germinated seeds was registered every day from the third day after sowing, after determining the percentage of germination, and it was calculated taking into account the number of germinated seeds, with respect to the initial number of seeds put to germinate.
Statistical analysis
The experimental design was completely randomized with 5 doses of water and each with 20 seeds. The data were subjected to a non-parametric statistical test by Kruskal-Wallis, to contrast the influence of the amount of irrigation and the number of germinated seeds. The correlation test was also applied to estimate the degree of association between temperature and pH of the substrate. The average temperature was 27.5°C and pH 7.6. The data were analyzed using INFOSTAT version 2018.
RESULTS
From 300 seeds sown of Polylepis incana, 17 seeds germinated in total, of this quantity nine seeds germinated with irrigation of residual water, four seeds germinated with irrigation of coconut water and four seeds germinated with irrigation of untreated water.
The seeds of P. incana, which were irrigated with wastewater and in a mixture of substrate that was black soil, sand and as fertilizer sheep manure, germinated 9 seeds which is equivalent to 9 % of a total of 100 seeds sown, while the seeds irrigated with coconut water and untreated, germinated four seeds (4 %), respectively (Figure 2).
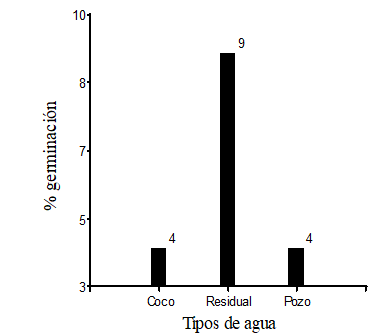
Figure 2 - The number of germinated seeds subjected to different types of water (coconut, residual and untreated)
The different amounts of water doses, applied for irrigation of seeds sown in black soil substrate, sand and as fertilizer sheep manure, did not present statistical differences in the germination of P. incana seeds (Hcalc (0,05) =0,83; P=0,85)
The analysis of correlation between temperature and pH, was of r=0.14, this implies a low correlation or affinity between these two dependent variables for the germination of the seed, therefore, the two variables can act indistinctly, without affecting the germination power of the P. incana (Figure 3). The temperature variations inside the greenhouse, had ranges of average of 22.67 to 31.13°C, being influential factors in the germination of seeds. The temperature of the substrate, where the P. incana seed was sown, which were irrigated with residual water, presented average records of 27.5°C and pH of 7.6.
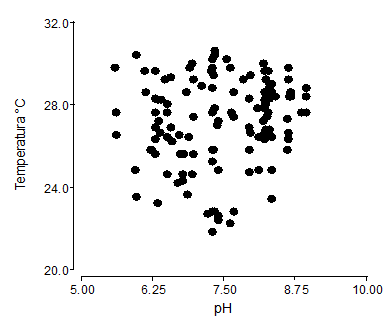
Figure 3 - Pearson's correlation of temperature (°C) and pH in substrates for the germination process of P. incana r= 0.14
DISCUSSION
The different doses of water, applied to the germination of seeds, do not influence the germination of P. incana seeds, however, the types of irrigation water, do influence the number of seeds germinated. The germination power of the plants is limited by reproductive difficulties due to genetic problems of the plant (Zutta et al., 2012), that is why in the treatment with coconut water, there was only a germination of 4 %, in the residual water of 9 % and in the untreated water of 4 %. These percentages coincide with that mentioned by (Argibay and Renison 2018), where it indicates that only 10 % of the seeds collected naturally are optimal for sowing and in addition the viability of germination, is also due to adverse climatic restrictions (Pulido and Ramos 2016) and the scarce dispersion in natural form (Wesche et al., 2008).
Likewise, it is important to mention that the temperature influences the germination of the P. incana (Delgado and Leon 2017) and these species that are above 4000 m a.s.l., are affected by the adversity of the low temperatures that are presented in the place (Simoes and Renison 2015). In the research, being inside a greenhouse was favorable for the germination percentage of P. incana seeds, because the temperature records fluctuated between an average range of 22.67 to 31.13°C. The optimal range for the germination of P. incana, according to our research, registers from 16.8 to 20.6°C, because when the average temperature of the day reached those ranges, the seeds react optimally and it also depends on the amount of irrigation that is done.
In the treatment with coconut water, there was a lower germination that was 4 %, despite presenting vitamins and chemical substances with promoting action on the germination of more than 50 % (Santos et al., 2013), however, in 100 % of the sown seeds, it was not favorable, it is likely that it can work in other species, because the coconut water was used to promote the germination of many species, such as carnation, because the coconut water presents amino acids and antioxidants (Arana-Paredes et al., 2015). According to Quinto et al., (2009), indicates that coconut water can be a promoter of germination, at temperatures between 16°C and 20°C (Arana-Paredes et al., 2015).
However, in our research, with the irrigation with coconut water, the temperatures inside the greenhouse oscillated from 22.67 to 31.13°C, for that reason, the coconut water, began to ferment, causing the formation of fungi around the seeds and this situation has been able to influence in the germination, mainly in those that were watered with 10, 15, 20, 25 ml; where there was not germination, however, with 5 ml, if there was germination.
In the treatment of wastewater there was a higher percentage of germination: from 9 %, it should be noted that the development of seeds depends on water quality parameters that influence the soil (Torres et al., 2008) because wastewater has a biochemical demand (Cardonell et al., 2012) and also has a large amount of nitrogen and phosphorus nutrients, mainly the water used, which was from the interior bay of Puno (Beltran et al., 2015) and in the seeds that were irrigated with 5ml daily, a better yield was obtained and a greater number of germinated seeds, but those that were irrigated with 10, 20, 25 ml, there was no germination, then, to smaller abundance of water within the treatments, there is greater number of germinated seeds. According to Enrico et al., (2004) plants have the capacity to retain and capture water in their organism, this shows that for P. incana, with a minimum amount of water the seed can germinate.
In this research, it was possible to obtain a 9 % germination power of P. incana seeds, while in natural form, they are only viable up to 3 % (Domic et al., 2017). This situation is encouraging for our results, because the native species have a low level of regeneration (Gutiérrez and Becerra 2018).
With the results obtained from this research, we provide knowledge so that public and private institutions can promote the production of P. incana seedlings from the germination of seeds, irrigated with treated wastewater, as this water is still suitable for irrigation, because it contains nitrogen and phosphorus. Then, the seedlings can be transplanted in their natural habitat, avoiding their diminishing process of the relict forests of Polylepis in the Peruvian high Andean region.
CONCLUSIONS
It has been registered a 9 % of germination power of the seeds of Polylepis incana sowed in the treatment with irrigation of residual water, being minor the seeds germinated with irrigation of coconut and untreated water.
At an irrigation supply of 5 ml, there is a greater number of germinated seeds, therefore, Polylepis seeds do not need abundant water for their development.
There is a low degree of affinity between the temperature and pH of the substrate, this implies no dependence on these parameters for the germination of the seed of P. incana.
ACKNOWLEDGEMENTS
To the Office of Environmental Management of the National University of the Altiplano of Puno, for the facilities provided for the realization of this study and to the laboratory of Ecology of the Faculty of Biological Sciences; for facilitating the use of different equipment and materials. This study was supported by the Office of Environmental Management and funds for formative research of the Universidad Nacional del Altiplano de Puno.
REFERENCES
ABDELLAOUI, R., BOUGHALLEB, F., ZAYOUD, D., NEFFATI, M. y BAKHSHANDEH, E., 2019. Quantification of Retama raetam seed germination response to temperature and water potential using hydrothermal time concept. Environmental and Experimental Botany [en línea], vol. 157, pp. 211216. Disponible en doi: https://doi.org/10.1016/j.envexpbot.2018.10.014
ARANA-PAREDES, M., RENGIFO, S. y CHICO-RUIZ, J., 2015. Germinación in vitro de Dianthus caryophyllus en diferentes medios de cultivo. Sagasteguiana [en línea], vol. 3, no. 1, pp. 5566. Disponible en: http://revistas.unitru.edu.pe/index.php/REVSAGAS/article/view/2009/1922
ARGIBAY, D. y RENISON, D., 2018. Efecto del fuego y la ganadería en bosques de Polylepis australis (Rosaceae) a lo largo de un gradiente altitudinal en las montañas del centro de la Argentina. Bosque [en línea], vol. 39, no. 1, pp. 145-150. Disponible en doi: https://doi.org/10.4067/S0717-92002018000100145
ARGOLLO, J., SOLIZ, C. y VILLALBA, R., 2004. Potencialidad dendrocronológica de Polylepis tarapacana en los Andes Centrales de Bolivia. Ecología en Bolivia [en línea], vol. 39, no. 1, pp. 5-24. Disponible en: http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S1605-25282004000700002
BELTRÁN, D., PALOMINO, R., MORENO, E., PERALTA, C., y MONTESINOS, D., 2015. Calidad de agua de la bahía interior de Puno, lago Titicaca durante el verano del 2011. Revista Peruana de Biología [en línea], vol. 22, no. 3, pp. 335-340. Disponible en doi: http://dx.doi.org/10.15381/rpb.v22i3.11440
CAPRILES, J. y FLORES, E., 2002. The economic, symbolic, and social imporatnce of the "keñua" (Polylepis spp.) during prehispanic times in the andean highlands of Bolivia. Ecotropia [en línea], vol. 8, pp. 225-235. Disponible en: http://saberesbolivianos.com/investigadores/Capriles/Capriles%20&%20Flores%202002.pdf
CARDONELL, M., FLÓREZ, M., MARTÍNES E. y PRIETO, A., 2012. Germinación de pratenses regadas con agua residual depurada. Ingeniería de Recursos Naturales y del Ambiente [en línea], vol. 11, pp. 73-82. Disponible en: http://www.redalyc.org/articulo.oaid=231125817011
CASTAÑEDA, R. y ALBÁN, J., 2016. Importancia cultural de la flora silvestre del distrito de Pamparomás, Ancash, Perú. Ecología Aplicada [en línea], vol. 15, no. 2, pp. 151-169. Disponible en doi: http://dx.doi.org/10.21704/rea.v15i2.755
CASTRO, A. y FLORES, M., 2015. Caracterización de un bosque de queñual (Polylepis spp.) ubicado en el distrito de Huasta, provincia de Bolognesi (Ancash, Peú). Ecología Aplicada [en línea], vol. 14 no. 1, pp. 1-10. Disponible en: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1726-22162015000100001
DAUD, A., HABIB, N. y RIERA, A., 2007. Actividad diurética de extracto acuoso de Polylepis australis (queñoa) y Phrygilanthus acutifolius (corpo). Estudio comparativo en ratas. Farmacología y Actividad Biológica [en línea], vol. 6, no. 6, pp. 337-339. Disponible en: https://www.redalyc.org/articulo.oa?id=85617472013
DEL POZO J.C., LOPEZ-MATAS, M.A., RAMIREZ-PARRA, E. y GUTIERREZ, C., 2005. Hormonal control of the plant cell cycle. Plant Physiol [en línea], vol. 123, no. 2, pp. 173-183. Dosponible en doi: https://doi.org/10.1111/j.1399-3054.2004.00420.x
DELGADO, J. y LEÓN-VARGAS, Y., 2017. Musgos (Bryophyta) de bosques de Polylepis sericea (Rosaceae) del estado Mérida (Venezuela). Boletín De La Sociedad Argentina De Botánica [en línea], vol. 52, no. 2,pp. 295313. Disponible en doi: https://doi.org/10.31055/1851.2372.v52.n2.17445
DOMIC, A. y CAPRILES, J., 2009. Allometry and effects of extreme elevation on growth velocity of the Andean tree Polylepis tarapacana Philippi (Rosaceae). Plant Ecology [en línea], vol. 205, no. 2, pp. 223234. Disponible en doi: http://dx.doi.org/10.1007/s11258-009-9612-5
DOMIC, A., MAMANI E. y CAMILO, G., 2013. Fenologia reproductiva de la kewiña (Poylepis tomentella, Rosaceae) en la puna semihúmeda de Chuquisaca (Bolivia). Ecologia en Bolivia [en línea], vol. 48, no. 1, pp. 3145. Disponible en: http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S1605-25282013000100004
DOMIC, A., PALABRAL-AGUILERA, A., GÓMEZ, M., HURTADO, R., ORTUÑO N. y LIBERMAN, M., 2017. Polylepis incarum (Rosaceae) una especie en peligro crítico en Bolivia: Propuesta de reclasificación en base al área de ocupación y estructura poblacional. Ecología en Bolivia [en línea], vol. 52, no. 2, pp. 116-131. Disponible en: http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S1605-25282017000200006
ENRICO, L., FUNES, G. y CABIDO, M., 2004. Regeneration of Polylepis australis Bitt. in the mountains of central Argentina. Forest Ecology and Management [en línea], vol. 190, no. 23, pp. 301309. Disponible en doi: https://dx.doi.org/10.1016/j.foreco.2003.10.020
GUTIÉRREZ, I. y BECERRA, P., 2018. Composición, diversidad y estructura de la vegetación de bosques ribereños en el centro sur de Chile. Bosque [en línea], vol. 39, no. 2, pp. 239-253. Disponible en doi: http://doi.org/10.4067/S0717-92002018000200239
HIDALGO, F., BUSTAMANTE, V., MUÑOZ, F., SERRA, M., RIOSECO, T. y CARDOZO, C., 2013. Estructura de una población de queñoa (Polylepis tarapacana Phil), Carcanal de Ujina, región de Tarapacá, Chile. Geobiota, 2pp
KESSLER, M. y SCHMIDT-LEBUHN, A., 2006. Taxonomical and distributional notes on Polylepis (Rosaceae). Organisms Diversity and Evolution [en línea], vol. 6, no. 1, pp. 6769. Disponible en doi: https://doi.org/10.1016/j.ode.2005.04.001
LANDI, M. y RENISON, D., 2010. Forestación con Polylepis australis en suelos erosionados de las Sierras Grandes de Córdoba: evaluación del uso terrazas y vegetación nodriza. Ecología Austral [en línea], vol. 20, pp. 47-55. Disponoble en: https://ri.conicet.gov.ar/handle/11336/53237?show=full
MENDOZA, W. y CANO, A., 2011. Diversidad del género Polylepis (Rosaceae, Sanguisorbeae) en los andes peruanos. Revista Peruana de Biologia [en línea], vol. 18, no. 2, pp. 197-200. Disponible en: http://www.scielo.org.pe/scielo.php?pid=S1727-99332011000200011&script=sci_abstract
MONTESINOS-TUBÉE, D., PINTO, A., BELTRÁN, D. y GALIANO, W., 2015. Vegetación de un bosque de Polylepis incarum (Rosaceae) en el distrito de Lampa, Puno, Perú. Revista Peruana de Biologia [en línea], 22(1): 8796. Disponible en doi: https://doi.org/10.15381/rpb.v22i1.11125
OLIVERA, M., CARVALHO, D., GOMES, D., PEREIRA, F. y MEDICI, L., 2018. Production of cut sunflower under water volumes and substrates with coconut fiber Produção de girassol de corte sob volumes de água e substratos com fibra de coco. Revista Brasileira de Engenharia Agrícola e Ambiental [en línea], vol. 22, no. 12, pp. 859865. Disponible en doi: http://dx.doi.org/10.1590/1807-1929/agriambi.v22n12p859-865.
PASSARINI, K., GAMARRA, F., VANALLE, R. y SANTANA, J., 2012. Reutilización de las aguas residuales en la irrigación de plantas y en la recuperación de los suelos. Información Tecnológica [en línea], vol. 23, no. 1, pp. 57-64. Disponible en doi: http://dx.doi.org/10.4067/S0718-07642012000100007
PATIÑO, C., MOSQUERA, F. y TULIO, G. 2011. Efecto inductor del agua de coco sobre la germinación de semillas y brotamiento de los cormos de la hierba de la equis (Dracontium ryumianum). Acta Biológica Colombiana [en línea], vol. 16, no. 1, pp. 133-142. Disponible en: http://www.redalyc.org/articulo.oa?id=319027887010
PULIDO, K. y RAMOS, C., 2016. Efecto de borde en la distribución de líquenes y el contenido de clorofilas en fragmentos de Polylepis quadrijuga (Rosaceae) en el páramo de La Rusia (Boyacá-Colombia). Revista de Biología Tropical [en línea], vol. 64, no. 4, pp. 1683-1697. Disponible en doi: http://dx.doi.org/10.15517/rbt.v64i4.22735
QUINTO, L., MARTÍNEZ-HERNÁNDEZ, P.A., PIMENTEL-BRIBIESCA, L. y RODRÍGUEZ-TREJO, D.A., 2009. Alternativas para mejorar la germinación de semillas de tres árboles tropicales. Revista Chapingo. Serie ciencias forestales y del medio ambiente [en línea], vol. 15, no. 1, pp. 23-28. Disponible en: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007 -40182009000100003
RENISON, D. y CINGOLANI, A., 1998. Experiencias en germinación y reproducción vegetativa aplicados a la reforestación con Polylepis australis (Rosaceae) en las Sierras de Córdoba, Argentina. Agriscientia [en línea], vol. 15, pp. 47-53. Disponible en: https://revistas.unc.edu.ar/index.php/agris/article/view/2607
RENISON, D., HENSEN, I. y CINGOLANI, A., 2004. Anthropogenic soil degradation affects seed viability in Polylepis australis mountain forests of central Argentina. Forest Ecology and Management [en línea], vol. 196 no. 2-3, pp. 327-333. Disponible en doi: https://doi.org/10.1016/j.foreco.2004.03.025
ROSERO, S., ARCOS, J., GUALLPA, M. y GUARACA, F., 2018. Efecto de la aplicación de solución nutritiva para el crecimiento inicial de Polylepis racemosa a nivel de vivero. Enfoque UTE [en línea], vol. 9, no. 2, pp. 198-207. Disponible en: 10.29019/enfoqueute.v9n2.207 (consultado junio de 2019)
SANTOS, J., BISPO, V., FILHO, A., PINTO, I., DANTAS, L., VASCONCELOS, D., ABREU, F., MELO, D., MATOS, I., FREITAS, F., GOMES, O., MEDEIROS, M. y MATOS, H., 2013. Evaluation of chemical constituents and antioxidant activity of coconut water (Cocus nucifera L.) and caffeic acid in cell culture. Anais da Academia Brasileira de Ciencias [en línea], vol. 85, no. 4, pp. 1235-1246. Disponible en doi: http://dx.doi.org/10.1590/0001-37652013105312
SCHMIDT-LEBUHN, A., KUMAR M. y KESSLER, M., 2006. An assessment of the genetic population structure of two species of Polylepis Ruiz y Pav. (Rosaceae) in the Chilean Andes. Flora [en línea], vol. 201, no. 4, pp. 317-325. Disponible en doi: https://dx.doi.org/10.1016/j.flora.2005.07.007
SELTMANN, P., RENISON, D., COCUCCI, A., HENSEN, I. y JUNG, K., 2007. Fragment size, pollination efficiency and reproductive success in natural populations of wind-pollinated Polylepis australis (Rosaceae) trees. Flora [en línea], vol. 202, no.7, pp. 547-554. Disponible en doi: https://doi.org/10.1016/j.flora.2006.12.002
SIMOES, N. y RENISON, D., 2015. ¿Cuántos años monitorear el éxito de plantaciones con fines de restauración?: Análisis en relación al micrositio y procedencia de las semillas. Bosque [en línea], vol. 36, no. 2, pp. 315-322. Disponible en doi: https://doi.org/10.4067/S0717-92002015000200016
TORRES, R., RENISON D., HENSEN I., SUAREZ R. y ENRICO L. 2008. Polylepis australis' regeneration niche in relation to seed dispersal, site characteristics and livestock density. ScienceDirect, vol. 254, pp. 255-260. Disponible en doi:10.1016/j.foreco.2007.08.007
VASCO, S., 2010. Tratamiento para promover la germinación de semillas de Polylepis reticulata Hieron y Polylepis lanuginosa Kunth. Universidad del Azuyo. Facultad de Ciencia y Tecnología. Disponible en: http://dspace.uazuay.edu.ec/handle/datos/162
VEGA, C., VELLEGAS, C., ROCABADO, P., QUEZADA, J., LÓPEZ, M. y QUEVEDO, A., 2018. Biología reproductiva de tres especies de Polylepis (P. neglecta, P. incarum y P. pacensis), con énfasis en su comportamiento germinativo. Ecología Austral. [en línea], vol. 28, pp. 310-324. Disponible en doi: https://doi.org/10.25260/EA.18.28.1.1.703
VEGA-KRSTULOVIC, C., BERMEJO-FRANCO, J., VILLEGAS-ALVARADO, G., QUEZADA-PORTUGAL, J., AGUILAR-LLANOS M. y CONDE-VELASCO, E., 2007. Propagación masiva de Polylepis tomentella Weddell ssp . nana mediante técnicas de cultivo in vitro. Ecología en Bolivia [en línea], vol. 39, no. 2, pp. 102-120. Disponible en: http://www.scielo.org.bo/scielo.php?script=sci_abstract&pid=S1605-25282007000800003&lng=pt&nrm=iso&tlng=es
WESCHE, K., CIERJACKS, A., ASSEFA, Y., WAGNER, S., FETENE, M. y HENSEN, I., 2008. Recruitment of trees at tropical alpine treelines: Erica in Africa versus Polylepis in South America. Taylor & Francis [en línea], vol. 0874, no. 1, pp. 35-46. Disponible en doi: https://doi.org/10.1080/17550870802262166
ZUTTA, B., RUNDEL, P., SAATCHI, S., CASANA, J., GAUTHIER, P., SOTO, A., VELAZCO, Y. y BUERMANN, W., 2012. Prediciendo la distribución de Polylepis: bosques Andinos vulnerables y cada vez más importantes. Revista Peruana de Biología [en línea], vol. 19, no. 2, pp. 205-212. Disponible en: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1727-99332012000200013
Conflict of interests:
The authors declare not to have any interest conflicts.
Authors' contribution:
The authors have participated in the writing of the work and analysis of the documents.
![]()
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0
International license
Copyright (c) 2020 Ángel Canales Gutiérrez, Yanina Ruth Huarasa Vilca