
Revista Cubana de Ciencias Forestales. 2020; September-December 8(3): 478-494
Translated from the original in spanish
Storage methods For Cinchona Pubescens Vahl. Seeds, Imbabura, Ecuador
Métodos de almacenamiento de semillas Cinchona Pubescens Vahl., Imbabura, Ecuador
Métodos de armazenamento de sementess Cinchona Pubescens Vahl., Imbabura, Equador
Jorge Luis Cué García1*
![]() https://orcid.org/0000-0002-4156-1555
https://orcid.org/0000-0002-4156-1555
Yajaira Alexandra Cuasque Peñafiel1![]() https://orcid.org/0000-0001-5822-7521
https://orcid.org/0000-0001-5822-7521
Hugo Vinicio Vallejos Álvarez1![]() https://orcid.org/0000-0003-3232-2381
https://orcid.org/0000-0003-3232-2381
Mario José Añazco Romero1
![]() https://orcid.org/0000-0003-4393-6485
https://orcid.org/0000-0003-4393-6485
Walter Armando Palacios Cuenca1
![]() https://orcid.org/0000-0002-0350-0399
https://orcid.org/0000-0002-0350-0399
Andrés Manolo Carrión Burgos1
![]() https://orcid.org/0000-0003-2021-8157
https://orcid.org/0000-0003-2021-8157
1Universidad Técnica del Norte. Ecuador.
*Correspondence author: jlcuegarcia@yahoo.com
Received: August 2nd, 2020.
Approved: September 20th, 2020.
RESUMEN
El manejo de semillas forestales en Ecuador, en relación al almacenamiento de las mismas, es todavía insuficiente, en particular la Cinchona pubescens Vahl. El objetivo fue determinar la calidad de las semillas y los mejores métodos de almacenamiento de semillas de C. pubescens, con el empleo de diferentes tiempos, envases y condiciones. La recolección de semillas fue en Intag, Imbabura. Los factores en estudio fueron: tiempo de almacenamiento, tipos de envase y condiciones de almacenamiento. Se determinó la calidad de las semillas al momento de la cosecha y en cada tratamiento. La pureza alcanzó un 76,5 %, el peso de 1 000 semillas fue 0,31 g y el contenido de humedad un 14 %. En el primer ensayo de almacenamiento no se manifestó la germinación en ningún tratamiento desde el primer mes. En el segundo ensayo, el mejor método de almacenamiento fue funda transparente almacenada en refrigeración durante una semana, el que mostró 9,25 % de poder germinativo. Las semillas de C. pubescens, pierden de manera rápida su viabilidad, que se hace nula al mes de almacenamiento.
Palabras clave: Refrigeración; Envases; Germinación; Pureza; Contenido de humedad; Vigor germinativo.
ABSTRACT
The management of forest seeds in Ecuador, in relation to their storage, is still insufficient, in particular the Cinchona pubescens Vahl. The objective was to determine the quality of the seeds and the best methods of seed storage of C. pubescens, using different times, containers and conditions. Seed collection was in Intag, Imbabura. The factors under study were: storage time, types of packaging and storage conditions. The quality of the seeds was determined at the time of harvest and in each treatment. Purity reached 76.5 %, the weight of 1,000 seeds was 0.31 g and the humidity content was 14 %. In the first storage trial, no germination was observed in any treatment from the first month. In the second trial, the best storage method was transparent cover stored in refrigeration for one week, which showed 9.25 % germination power. Cinchona pubescens seeds lose their viability quickly, which becomes null after one month of storage.
Keywords: Refrigeration; Containers; Germination; Purity; Humidity content; Germinative vigor.
RESUMO
O manejo de sementes florestais em Equador, em relação a armazenagem das mesmas, é ainda insuficiente, particularmente, a Cinchona pubescens Vahl. O objetivo foi determinar a calidade das sementes e os melhores métodos de armazenagem delas, com o emprego de diferentes tempos, envases e condições. A recoleita de sementes realizou se em Intag, Imbabura. Os fatores em estudo foram: tempo de armazenagem, tipos de envases e condições de armazenagem. Se determinou a calidade das sementes no momento de semear e em cada tratamento. A pureza alcanzou um 76,5 %; A pesagem de 1 000 sementes foi 0,31g e o conteúdo da umidade, um 14 %. No primeiro ensaio de armazenagem não foi manifesto em refrigeração tratamento nenhum do primeiro mês, no caso da germinação, mas num segundo ensaio, a funda transparente foi o melhor método do ensaio, armazenadas durante uma semana, o que mostrou 9.25 % de poder germinativo. As sementes de C. pubescens, perdem com rapidez sua viabilidade a que se faz nula ao mês da armazenagem.
Palavras clave: Refrigeração; Envases; Germinação; Pureza; Conteúdo de umidade; Vigor germinativo.
INTRODUCTION
The genus Cinchona, of the botanical family Rubiaceae, known as "cascarilla or cinchona tree" is made up of 23 species (Andersson and Taylor, 1994). It is native to the South American Andes (Garmendia, 2005). It is distributed along the tropical and equatorial zone of the Andes, from 10° North latitude to 20° South latitude. In Ecuador there are 12 of these species, (Ulloa and Jørgensen, 1995), where four are endemic and eight are native. Generally, they are medium to small size trees or shrubs with bitter bark, they can reach a height that can reach 25 meters (Mahecha, et al., 2016). In natural conditions the genus Cinchona presents low rate of germination and regeneration, found only in remote places and in small groups (Buddenhagen, et al., 2004).
The species C. pubescens (quinine tree, red quina, cascarilla, quina) is an evergreen tree, 10-25 m high, with a diameter at breast height (DBH) of 20-80 cm in Ecuador (Jugar, 2015). It has the largest geographical distribution area within its genus in the Americas and is found from northern Bolivia to Costa Rica. It is introduced in Tahiti and Hawaii, Asia and in Tanzania, Africa. This species grows at altitudes between 300 and 3300 m (Jäger, 2015), very robust so it was used as a grafting pattern. The total alkaloid content is 3.8 %, of which less than 50 % is quinine. This species is considered rare and endangered in its native range in Ecuador (Günter et al., 2004), while (Jäger and Kowarik, 2010) and (Jäger, 2015), it is considered invasive in the insular conditions of Galapagos.
In the zone of Intag, Imbabura Ecuador, the species C. pubescens is located in relict forests, secondary forests and forming part of silvopastoral systems, at altitudes higher than 1 600 and up to 3 000 m above sea level with a scarce abundance since, according to the inhabitants of the place, its trees are cut down for timber, coinciding with (Gómez, 2016), which poses a similar situation for the District of Kañaris, Lambayeque Region, in Peru.
In the study of forest germplasm supply chains in Ecuador (Prado, Samaniego and Ugarte, 2010), it is stated that there is little availability of quality forest seed in quantity, timely supply to meet producer demand. They also state that there is limited technical and scientific information on the techniques for producing, processing and storing forest seeds of many native species to ensure their viability.
The techniques for the collection, processing and storage of seeds, is a basic condition for designing programs of reforestation, ecological restoration and agroforestry development. Ceballos and López (2007) state that there are few studies on native species, such as the case of the C. pubescens species, on phenological calendars and on the collection and processing (cleaning, drying, humidity content and storage) of forest seeds in Colombia, which is the case in Ecuador. Given the adaptability of this species to the conditions of Intag and other areas of the country, it can be used for various purposes within the country's forestry programs.
Stored seeds are a primary means of production in a country's plant production programs; however, seeds cannot retain their germination capacity indefinitely. The maintenance of their viability depends very much on the storage conditions (Doria, 2010).
In general, the heterogeneity of forest seeds does not allow the same storage technique to be approved, since many show good storage behaviour, while others, on the contrary, deteriorate rapidly under the same conditions. The management of the relative humidity of the air and the temperature of the environment are two key factors to achieve the best results in the storage of the seeds, since they directly influence the speed of breathing of the same ones (Blanco, Durañona and Acosta, 2016).
The cascararilla had a use in the past for its contribution with the quinine alkaloid to act on malaria. The most used species was C. officinalis, while the first species used was C. pubescens. On this basis of knowledge, chemical was synthesized. The virus that causes malaria has mutated and therefore new synthetic products are required, but it is also necessary to know the chemical compounds of other species of Cinchona, so the study of C. pubescens. It is necessary then, to know different aspects of its physiology, among others: time of fructification, viability of the seeds and the conditions of storage that allow to have a successful germination of the same ones.
The objective was to determine the quality of the seeds and the best method(s) to store the seeds of C. pubescens, collected in the area of Intag, Northwest of the Ecuadorian Andes.
MATERIALS AND METHODS
The collection of fruits was carried out in the town of Pucará Alto, Intag, Imbabura, Ecuador, where 30 individuals were selected at random as candidate trees in a silvopastoral system, which were marked and georeferenced. The phenology of the species was carried out through direct observation in the field and the most adequate moment for the collection of the fruits was determined, which was carried out in the month of September, taking into account the relationship of the color of these with their state of ripeness, which corresponded to the color from maroon to brown.
The drying was done in ambient conditions under shade, during three days, for which they were placed on a cloth and constantly removed to obtain a homogeneous drying. The process of extracting the seeds was done manually from fruits selected according to the best phytosanitary condition and of greater length and width. The extracted seeds were dried, by a process similar to the fruits in environmental conditions on a laboratory table. The seeds were partially cleaned, by means of a manual procedure, eliminating the remains of larger fruits.
The ISTA Standards (2016) were used to determine the quality of the seeds in terms of purity, weight of 1000 seeds, humidity content and germination power.
Three factors were studied: type of containers, storage medium and time. The levels by factors were:
There were 48 treatments evaluated for the first trial and 32 treatments for the second trial, using an unrestricted randomized design, under laboratory conditions.
The observations on germination were made during 40 days, at 10:00 a.m.
Germination power (Equation 1).
![]()
Where:
PG: Germination power;
Sg: Sprouted seeds;
Ts: Total number of seeds sown
Germinative vigor (Equation 2).
![]()
Where:
VM: maximum or top value that is presented between the values product of the division of
the accumulated percentage of germination and the number of days that it took to obtain it.
GDM: is the average daily germination, calculated as the ratio between the final percentage
of germination (PG) and the number of days it took to reach that value.
The assumptions for carrying out the analysis of trifactor variance were verified, which were not fulfilled, and a non-parametric Kruskal-Wallis test was carried out.
RESULTS
The collected seed showed a purity of 75.8 %, which can be given by the type of fruit that is a dehiscent capsule, reducing impurities from the process of drying the fruit and at the time of extraction. It was obtained a weight of 0.315 g for 1 000 seeds of C. pubescens, while the percentage of humidity content of the seeds was 13.6 %. The germinative power was very low, 12 %, the beginning of germination at 29 days and culminated at 36 days, while the value of 0.43 for germinative vigor was negligible.
Storage tests
In the first germination test on C. pubescens seeds, after one month of storage, zero percentage of germination was obtained, for the environments and types of containers used.
For the second test with weekly evaluation periods it is obtained that, the assumptions of homogeneity and normality for the results of the germination power are not fulfilled (Figure 1 and Figure 2), which shows a fan shape in the distribution of the residuals of germination.
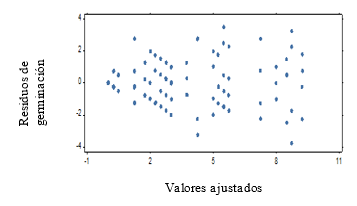
Figure 1. - Diagram of homogeneity of the germination power of C. pubescens seeds
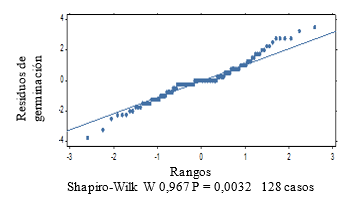
Figure 2. - Normal probability diagram for C. pubescens seed germination
The normality graph of the germination power data (Figure 2), plus the results of the Shapiro-Wilk test at P < 0.05, indicate the non-fulfillment of the normality assumption. The Kruskal-Wallis test (Table 1) shows significant differences between treatments (P = 0.002).
Table 1. - Germination power of treatments
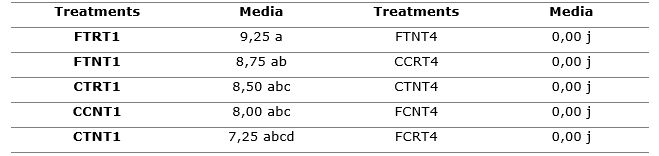
Types of containers: CT- translucent glass; CC- amber glass; FT- translucent plastic cover; FC- black plastic cover; R- refrigeration; N- ambient; T1- one week and T4- four weeks
Different letters in the rows and columns indicate significant differences for P < 0.05
The treatment with transparent sleeves in refrigeration for one week, with 9.25 % germination, was the best treatment (Table 1), while those with the worst behavior correspond to the treatments of week four, regardless of the medium and the type of container, since they show zero percentages of germination.
By eliminating the treatments of week four of storage, the assumptions of homogeneity and normality for the results of the germination power are not fulfilled (Figure 3 and Figure 4), which shows a fan shape in the distribution of the residuals of germination.
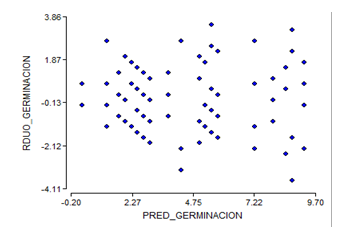
Figure 3. - Diagram of germination power homogeneity of C. pubescens seeds for three weeks of storage
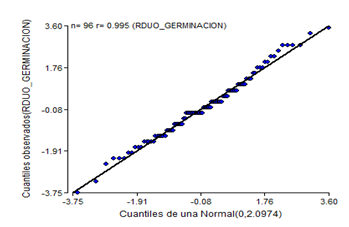
Figure 4. - Normal probability diagram of the germination power of C. pubescens seeds for three weeks of storage
The normality graph of the germination power data at three weeks (Figure 4), plus the results of the Shapiro- Wilk test at P < 0.05, indicate that the normality assumption was not fulfilled.
The Kruskal-Wallis test shows significant statistical differences between the treatments for the three weeks of storage with respect to the variable germination power. The mean comparison test (Table 2) shows differences between treatments. The treatment of transparent sheath in refrigeration is ratified at week one within the best group, to which the treatments of transparent sheath in natural medium and translucent glass in refrigeration are also incorporated, both also for the first week.
Table 2. - Power and germinative vigor of treatments for three weeks' storage
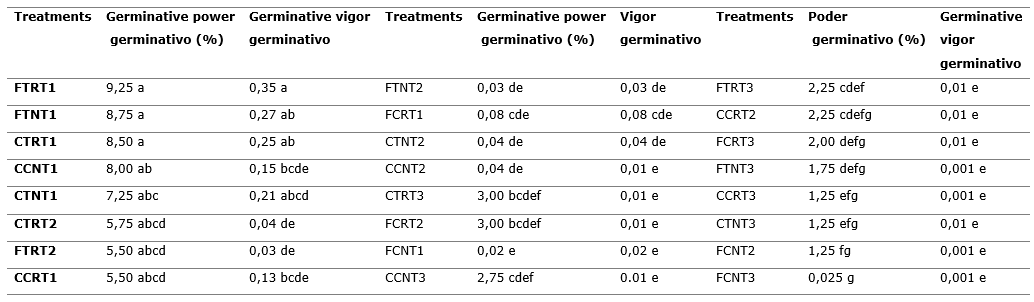
Types of Legend: Containers: CT- translucent glass; CC- amber glass; FT- translucent plastic cover; FC- black plastic cover; R- refrigeration; N- ambient; T1- one week; T2- two weeks; T4- three weeks; T4- four weeks Kruskal Wallis at P <0.01.
Different letters in the rows and columns indicate significant differences for P < 0.05
The results of the normality and homogeneity tests for germinative vigour showed a similar tendency to germination power. Both tests are not fulfilled (Figure 5 and Figure 6), where it is observed with typical cone for homogeneity and normality ratifies its non fulfilment according to the Shapiro-Wilk test at P < 0.01.
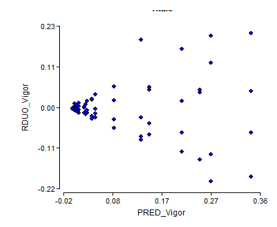
Figure 5. - Diagram of germinative vigor homogeneity of C. pubescens seeds for three weeks of storage
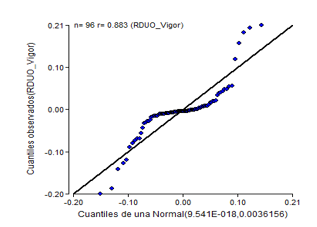
Figure 6. - Normal probability diagram of germinative vigor of C. pubescens seeds for three weeks of storage
The results of the comparison of means for germinative vigor (Table 2) show that there is no biunivocal relationship between the order of treatments with respect to germinative power. The best treatment is the refrigerated translucent sheath treatment after one week, but the group in which the treatments are located changes with respect to the grouping of the averages for germination power.
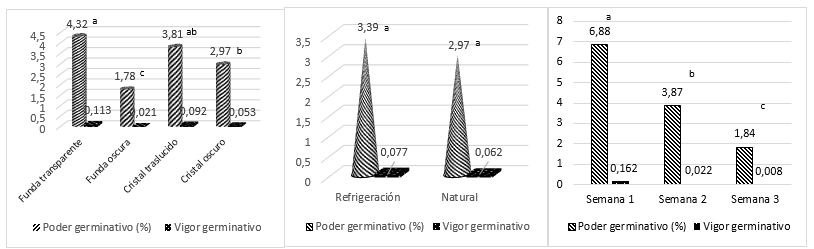
Figure 7. - Power behavior and germinative vigor of the factors under study
Different letters indicate significant differences for P < 0.05
When comparing the averages of the study factors for germination power and vigor, Figure 7, it is observed that the translucent containers are superior to the dark colored ones where the transparent cover stands out, while there are no significant differences between the averages and week one reaches a higher average than the rest, being week three the one with the worst behavior.
DISCUSSION
In the experience, 77.1 % purity was obtained in C. officinalis, while (Caraguay et al., 2016), it reached 38.04%, in the same species. This result is given by the benefit process carried out from the selection of the fruits of greater size and better sanitary state, plus the handling of the seeds from the extraction of the fruits and its initial cleaning, all of which allowed to have a seed with less impurities.
The weight of 1,000 seeds obtained, is greater than that posed (Campos et al., 2014) of 0.024 g. We agree with these authors that this behavior could be one of the disadvantages of germination, because when the seeds are smaller they have minimum reserves (Pascualides and Ateca, 2013; Alvarado et al., 2015; Rodríguez and Pompa, 2016; Ruíz, et al., 2018), which can make use of it quickly after the harvest, so it contributes little to the growth of the new plant. This constitutes a risk for the non-occurrence of germination, since this so complex process can be initiated from the phase of imbibition, without necessarily occurring the activation of the synthesis and degradation, leading to the division and cellular elongation, so that the rupture of the seminal cover by the embryo occurs.
The result of humidity in the seeds, 13.6 %, is lower than that found by (Campos et al., 2014), with a value of 16.67% in the same species. The initial humidity content can influence the period of imbibition of the seed (Hernández et al., 2018), which implies a greater speed in the absorption of water for seeds with lower humidity content. This greater speed of absorption at the beginning of the imbibition does not imply a superior behavior in the germination of the seeds (Vargas et al., 2015), that when studying two contents of humidity of the seed in four species (S. saman, 11.5 and 6.1 %; P. dulce, 13.8 and 5.5 %; J. caucana, 8.4 and 3.5 %; T. rosea, 8.3 and 3.6 %) does not obtain significant differences for the germination between the levels of initial humidity of the seeds for each species. On the other hand (Lines et al., 2006), they evaluated five moisture contents (4.8 %, 10.5 %, 21.3 %, 26.3 % and 40.3 %) and found that the seed germinates in less time and greater percentage with the highest humidity content and that the decrease in the germination percentage is proportional to the decrease in the humidity content.
A greater time of drying of the seeds in reason of obtaining a smaller content of humidity in these, can affect its germinative capacity since, the absorption of humidity by the seeds to reach its hygroscopic balance during the process of imbibition, causes the deterioration of the plasmatic membrane of the seeds and diminishes its physiological quality (Crivelari, et al., 2019).
The behavior of the germinative power, 12 % was very low, which can be given by different causes, such as: undeveloped embryos, dead embryos, very small seeds and low germinative reserve, among others. The seeds of C. pubescens present difficulties in their germination, (Campos et al., 2014), since it is directly influenced by the physiological maturation of the seeds. A cause to be considered would also be the level of humidity of the seeds once the drying process is done, since a low percentage of humidity implies that the seed has a negative matric potential, so it tends to soak very quickly (phase I), regardless of whether the seed is dormant or viable (Matilla, 2008).
Small seeds with low reserves and low humidity, make a rapid absorption of water, which causes temporary alterations in the differential permeability of the membranes of the seed and, therefore, a loss to the surrounding environment of solutes and different metabolites of low molecular weight (sugars, organic acids, ions, amino acids, peptides, etc.), corroborated in the results of (Ribeiro et al., 2015). The results make it possible to assume as low the metabolic activation of the seeds, which prevents them from developing phase II (plateau), a period of delayed water absorption, without reaching the activation of the embryo. Dead and dormant seeds maintain this level of typical hydration of phase II, but unlike seeds germinating they do not enter phase III, which is associated with the protrusion of the radicle. Everything agrees with what was concluded by (Marler, 2019), since the speed of imbibition during the physical phase does not necessarily correlate directly with the speed of final germination.
In imbibition, seeds are transformed from an inactive dry state (without translation) to a fully active metabolic state, and selectively translate subsets of these stored RNAm. Therefore, the seeds provide a unique on/off switch (Sajeev et al., 2019), regulated by the development for translation. The results of the very low germination power of C. pubescens seeds in the study suggest that their activation could be reduced in seeds that failed to germinate, even when they developed phase I imbibition.
In relation to the maximum time of germination, 36 days, do not coincide with that obtained by (Bargali and Singh, 2007) which was 22 days, nor with Campos et al., (2014), Cinchona sp. from the town of La Cascarilla-Jaén, with original soil substrates, which fluctuated between 12 and 24 days, independently of the origin of the different places of the mentioned town.
The germinative vigour was negligible, 0.43, in accordance with the low germination power and the duration of germination. Those species that distribute the energy they devote to fructification in producing a large number of small seeds, as is the case of C. pubences, this capacity to be widely distributed because it has greater opportunities for the seeds to find a favorable place to grow (Rosseto et al., 2000). Its small size contributes little to the growth of the new plant, which has a high probability of dying because it depends quickly on the resources available in the environment. In addition, these plants are sensitive to damage from biotic and abiotic agents, and their survival is minimal, which is compensated by the large number of seeds that individuals of this species produce.
Storage tests
The non-germination of the seeds after one month of storage coincides with what was stated by Acosta (1945), referring to the fact that when the seeds are fresh they germinate between a period of 11 and 20 days and if they are old, depending on the storage period, the germination percentage decreases, therefore the seeds of this species when they are subjected to storage for more than one month, lose their viability.
The results show that the time factor influences the germination since, the seeds of C. pubescens when being submitted to each one of the treatments of conservation in times from 1 to 4 weeks, the percentage of germination diminishes as the time of conservation is increased until arriving at a value of zero to the fourth week, independently of the means of storage and the type of used package.
The treatments under refrigeration conditions for the different types of containers in the first week of storage showed a better trend in the germination behavior. Ortiz et al. (2004) highlight the advantage of air-conditioned chambers with domestic air conditioning, confirming the favorable effect of low temperatures in seed storage. On the other hand, Ruíz et al., (2017), Valverde et al., (2019), ratify that the refrigerated treatments showed significance with respect to the non-refrigerated treatments.
When studying the storage of Dypsis lutescens seeds, Doria et al., (2012), found that the best container was black polyethylene followed by translucent plastic bottles, while the best treatments were black polyethylene in refrigeration and environmental conditions. These results differ from those obtained in this research because the translucent polyethylene sleeves were the best behaved, but they coincide as they are the best containers in both storage conditions.
In relation to the light requirement, most tree species behave as indifferent (Flores et al., 2017), germinating under both light and dark conditions. Based on the fact that, ecologically, the perception of light by the seed can act as an indicator of the amount of light available to the seedling, it can indicate planting depth, canopy shading and soil disturbance. This behavior is supported by the fact that the presence or absence of light indicates to the seeds if they are close to the surface or buried, on the other hand the red/distant red ratio (R/RL) is an indicator of the presence and size of forest clearings, that is to say, of the density of the canopy according to what Escobar and Cardoso have exposed, (2015). In the experience, four of the five treatments with the best behavior have the characteristic of a translucent type of container, which allows us to assume that the seeds of C. pubencens could better manifest their germination, with certain levels of presence of light during storage, either artificial or natural in the soil seed bank.
By using airtight storage, containers that prevent air and humidity from entering the product. In these conditions, the breathing of the seed and the insects (when there are any) deplete the existing oxygen, causing the death of the latter and the reduction of the activity of the seed, so the storage can last a long time without deterioration.
When opening the containers to extract the seeds at the time of the germination assemblies, a gaseous exchange with the local environment is carried out. Once the container is closed, an exchange of humidity between the seeds and the environment inside the container is restored, which generally causes an increase in the humidity content of the seeds. This aspect is more relevant for storage in environmental conditions, which can accelerate the deterioration of the seeds, unlike the cold environment since the humidity in the microenvironment of the container is lower, since the relative humidity in the refrigerator is also lower (Ruíz et al., 2017).
The germinative vigour of the treatments shows a similar tendency to that of the germinative power, but it is distinguished that there are some increases for some cases, which is given by the reduction of the number of days in manifesting the maximum accumulated germinative power. In experience, values of less than one to zero are obtained, while Campos et al., (2014) achieved values from zero to 13.5 of germinative vigor.
CONCLUSIONS
The quality of the seeds of C. pubescens is low in relation to the percentage of germination, of low weight and average physical purity.
The germination of the seeds of C. pubescens does not occur after a month of storage, with a significant reduction from the first week, in both transparent covers and packaging is the best option, without any difference between the means of refrigeration and natural environment.
REFERENCES
ACOSTA SOLÍS, M., 1945. Botánica de las Cinchonas. Flora, vol. 6, no. 15-16, pp. 29-55.
ALVARADO VÁZQUEZ, M.A., FOROUGHBAKHCH, R., RÄHIM FOROUGHBAKHCH, GUZMÁN LUCIO,
M.A., ROCHA ESTRADA, A., HERNÁNDEZ PIÑERO, J.L., CÁRDENAS ÁVILA, M.L. y SOTO GARCÍA,
B.M., 2015. Efecto de la madurez del fruto, peso de la semilla y tiempo de almacenamiento en
la viabilidad y germinación de la semilla de candelilla (Euphorbia antisiphylitica Zucc.). Phyton [en línea], vol. 84, pp. 70-79. [Consulta: 15 febrero 2020]. Disponible en: https://www.researchgate.net/publication/317532447_Efecto_de_la_madurez_del_fruto_peso_de_la_semilla_y_tiempo_de_almacenamiento_en_la_viabilidad_
y_germinacion_de_la_semilla_de_candelilla_Euphorbia_antisiphylitica_Zucc
ANDERSSON, L; TAYLOR, C. 1994. Rubiaceae cinchoneae coptosapelteae. En: Harling G, Andersson L (Eds), Flora of Ecuador no 50. Council for nordic publications in Botany. Museo Botánico. 114p.
BARGALI, K. y SINGH, S.P., 2007. Germination behaviour of some leguminous and actinorhizal plants of Himalaya: Effect of temperature and medium. Tropical Ecology [en línea], vol. 48, no. 1, pp. 99-105. [Consulta: 23 noviembre 2019]. ISSN 0564-3295. Disponible en: https://www.researchgate.net/publication/238087379_Germination_behaviour_of_some_leguminous_and_actinorhizal_plants_of_Himalaya_Effect_of_temperature_and_medium.
BLANCO VALDÉS, Y., DURAÑONA, H. y ACOSTA ROCA, R., 2016. Efecto de la temperatura y la humedad en la conservación de granos de maíz en silos metálicos refrigerados. Cultivos Tropicales [en línea], vol. 37, no. 4, pp. 105-114. [Consulta: 21 de enero de 2020]. ISSN 0258-5936. DOI 10.13140/RG.2.2.13900.21127. Disponible en: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0258-59362016000400010&lng=es&nrm=iso&tlng=es.
BUDDENHAGEN, C.E., RENTERIA, J.L., GARDENER, M., WILKINSON, S.R., SORIA, M., YÁNEZ, P., TYE, A. y VALLE, R., 2004. The Control of a Highly Invasive Tree Cinchona pubescens in Galapagos1. En: container-title: Weed Technology, Weed Technology [en línea], vol. 18, no. sp1, pp. 1194-1202. [Consulta: 17 marzo 2020]. ISSN 0890-037X, 1550-2740. DOI 10.1614/0890-037X(2004)018[1194:TCOAHI]2.0.CO;2. Disponible en: https://bioone.org/journals/Weed-Technology/volume-18/issue-sp1/0890-037X(2004)018[1194:TCOAHI]2.0.CO;2/The-Control-of-a -Highly-Invasive-Tree-Cinchona-pubescens-in/10.1614/0890-037X(2004)018[1194:TCOAHI]2.0.CO;2.full.
CAMPOS RUÍZ, J., REBAZA DE CHICO, L.C. y CHICO RUÍZ, J., 2014. Efecto del ácido giberélico, nitrato de potasio y agua de coco en la germinación de semillas de quina, Cinchona pubescens. Revista REBIOLEST [en línea], vol. 2, no. 1, pp. 5-15. [Consulta: 27 de marzo 2020]. Disponible en: https://revistas.unitru.edu.pe/index.php/ECCBB/article/view/637.
CARAGUAY YAGUANA, K.A., ERAS GUAMAN, V.H., GONZÁLEZ ZARUMA, D., MORENO SERRANO, J., MINCHALA PATIÑO, J., YAGUANA ARÉVALO, M. y VALAREZO ORTEGA, C., 2016. Potencial reproductivo y análisis de calidad de semillas de Cinchona officinalis L., provenientes de relictos boscosos en la provincia de Loja-Ecuador. Revista Investigaciones Altoandinas [en línea], vol. 18, no. 3, pp. 271-280. ISSN 2306-8582. [Consulta: 18 de febrero de 2020] Disponible en: https://dialnet.unirioja.es/servlet/articulo?codigo=5645611.
CEBALLOS, A.J. y LOPEZ, J.A., 2007. Conservación de la calidad de semillas forestales nativas en almacenamiento. En: Accepted: 2012-04-03T21:33:57Z, Cenicafé [en línea], vol. 58, no. 4, pp. 265-292. [Consulta: 18 febrero de 2020]. ISSN 0120-0275. Disponible en: https://biblioteca.cenicafe.org/handle/10778/116.
CRIVELARI DA COSTA, P.M., BIANCHINI, A., CANEPPELE, C., AZEVEDO, P.H., SILVA, A.L. da, AZEVEDO DOS SANTOS, M. y XAVIER PEREIRA, P.S., 2019. Moisture Content and Packaging Condition on the germination of Amaranth BRS Alegria Seeds. Journal of Experimental Agriculture International [en línea], vol. 38, no. 4, pp. 1-8. [Consulta: 27 marzo 2020]. ISSN 2457-0591. DOI 0.9734/jeai/2019/v38i430305. Disponible en: https://www.researchgate.net/publication/334218032_Moisture_Content_and_Packaging_Condition_on_the_Germination_of_Amaranth_BRS_Alegria_Seeds.
DORIA GONZÁLEZ, J., BENÍTEZ FERNÁNDEZ, B. y SOTO, F., 2012. Influencia de diferentes métodos de conservación en la germinación de semillas de palma areca (Dypsis lutescens, H. Wendel). Cultivos Tropicales [en línea], vol. 33, no. 2, pp. 56-60. [Consulta: 28 julio 2020]. ISSN 1819-4087. Disponible en: https://www.researchgate.net/publication/262465901_Influencia_de_diferentes_metodos_de_conservacion_en_la_germinacion_de_semillas_de_palma_areca_Dypsis_lutescens_H_Wendel.
DORIA, J., 2010. Generalidades sobre las semillas: su producción, conservación y almacenamiento. Cultivos Tropicales [en línea], vol. 31, no. 1. [Consulta: 20 agosto 2020]. ISSN 0258-5936. Disponible en: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0258-5936201 00001 00011&lng=es&nrm=iso&tlng=es.
ESCOBAR, D.F. y CARDOSO, V.J.M., 2015. Germinación y latencia de semillas de miconia chartacea (Melastomataceae), en respuesta a luz, temperatura y hormonas vegetales. En: Accepted: 2018-12-11T16:41:40Z, Revista de Biologia Tropical [en línea], vol. 63, no. 4, pp. 1169-1184. [Consulta: 20 de agosto 2020]. ISSN 2215-2075. DOI 10.15517/rbt.v63i4.17955. Disponible en: https://repositorio.unesp.br/handle/11449/168533.
FLORES, P., POGGI, D., GARCÍA, S., CATRARO, M. y GARIGLIO, N., 2017. Ruptura de la dormición y exigencias de luz para la germinación de semillas de Juglans nigra. Revista FAVE-Ciencias Agrarias [en línea], vol. 16, no. 2, pp. 33-46. [Consulta: 27 marzo 2020]. ISSN 2346-9129. DOI 10.14409/fa.v16i2.7018. Disponible en: https://www.researchgate.net/publication/322086032_Ruptura_de_la_dormicion_y_exigencias_de_luz_para_la_germinacion_de_semillas_de_Juglans_nigra.
GARMENDÍA, S.A., 2005. El árbol de la quina (Cinchona spp.): distribución, caracterización de su hábitat y arquitectura [en línea]. Ecuador: Universidad Técnica Particular de Loja. ISBN 978-9978-09-574-4. [Consulta: 27 de marzo 2020]. Disponible en: https://books.google.com.cu/books/about/El_%C3%A1rbol_de_la_quina_Cinchona_spp.html?id=lnMgPQAACAAJ&redir_esc=y.
GÓMEZ SILVERA, A., BERAUN MACEDO, L.A., GÓMEZ RENGIFO, O.J. y LLATAS DUCEP, E., 2016. Procesos de regeneración natural de la quina o cascarilla (Cinchona spp.): en los bosques de neblina del distrito de Kañaris, región Lambayeque. INIA. Estación Experimental Agraria Vista Florida-Lambayeque [en línea], [Consulta: 20 agosto 2020]. Disponible en: http://repositorio.inia.gob.pe/bitstream/inia/572/1/Gomez-procesos_reg.pdf.
GÜNTER S., B. STIMM Y M. WEBER. 2004. Silvicultural contributions towards sustainable management and conservation of forest genetic resources in Southern Ecuador. Lyonia 6, 1: 75-91. [Consulta: 22 noviembre 2019]. Disponible en: http://www.lyonia.org/articles/rbussmann/article_307/pdf /article.pdf
HERNÁNDEZ ANGUIANO, L.A., LÓPEZ UPTON, J., RAMÍREZ HERRERA, C., ROMERO MANZANARES, A., HERNÁNDEZ ANGUIANO, L.A., LÓPEZ UPTON, J., RAMÍREZ HERRERA, C. y ROMERO MANZANARES, A., 2018. Variación en germinación y vigor de semillas de Pinus cembroides y Pinus orizabensis. Agrociencia [en línea], vol. 52, no. 8, pp. 1161-1178. [Consulta: 17 febrero 2020]. ISSN 1405-3195. Disponible en: http://www.scielo.org.mx/scielo.php?script=sci_abstract&pid=S1405-31952018000801161&lng=es&nrm=iso&tlng=es.
HERRERA, J., LINES, K. y VÁSQUEZ, W., 2006. Estudio de la germinación y la conservación de semillas de cedro maría (Calophyllum brasiliense). Revista Tecnología en Marcha [en línea], vol. 19, no. 1. [Consulta: 27 marzo 2020]. Disponible en: https://www.researchgate.net/publication/277198452_Estudio_de_la_germinacion_y_la_conservacion_de_semillas_de_cedro_maria_Calophyllum_brasiliense.
INTERNATIONAL SEED TESTING ASSOCIATION (ISTA). 2016. Reglas Internacionales para el Análisis de las Semillas. Zürichstr. 50, CH-8303 Bassersdorf, Suiza. Versión en español. [Consulta: 22 octubre 2018]. Disponible en: https://vri.umayor.cl/images/ISTA_Rules_2016_Spanish.pdf
JÄGER, H., 2015. Biology and Impacts of Pacific Island Invasive Species. 11. Cinchona pubescens (Red Quinine Tree) (Rubiaceae). Pacific Science [en línea], vol. 69, no. 2, pp. 133154. [Consulta: 20 julio 2020]. DOI 10.2984/69.2.1. Disponible en: https://www.researchgate.net/publication/281129770_Biology_and_Impacts_of_Pacific_Island_Invasive_Species_11_Cinchona_pubescens_Red_Quinine_Tree_Rubiaceae.
JÄGER, H. y KOWARIK, I., 2010. Resilience of native plant community following manual control of invasive Cinchona pubescens in Galápagos. Restoration Ecology [en línea], vol. 18, no. 1, pp. 103-112. [Consulta: 22 noviembre 2019]. ISSN 1526-100X. Disponible en: https://www.researchgate.net/publication/227724741_Resilience_of_Native_Plant_Community_Following_Manual_Control_of_Invasive_Cinchona_pubescens_in_Galapagos.
MAHECHA VEGA, G., OVALLE ESCOBAR, A., CAMELO SALAMANCA, D., ROZO FERNÁNDEZ, A. y BARRERO BARRERO, D., 2016. Vegetación del territorio CAR: 450 especies de sus llanuras y montañas [en línea]. Colombia: Corporación Autónoma Regional de Cundinamarca—CAR. ISBN 978-958-8188-06-5. [Consulta: 27 marzo 2020]. Disponible en: https://books.google.com.cu/books/about/Vegetaci%C3%B3n_del_territorio_CAR.html?id=wKvitgAACAAJ&redir_esc=y.
MARLER, T., 2019. Temperature and Imbibition Influence Serianthes Seed Germination Behavior. Plants [en línea], vol. 8, no. 4, pp. 107. DOI 10.3390/plants8040107. Disponible en: https://www.semanticscholar.org/paper/Temperature-and-Imbibition-Influence-Serianthes-Marler/a158bbae75e4742d13f6f42c427f32fe31c82031.
MATILLA, A.J., 2008. Desarrollo y germinación de las semillas. En: J. AZCÓN BIETO y M. TALÓN, Fundamentos de Fisiología Vegetal [en línea]. 2. S.l.: McGraw Hill, pp. 537-558. [Consulta: 22 noviembre 2019]. Disponible en: https://www.researchgate.net/publication/271512205_Desarrollo_y_germinacion_de_las_semillas.
ORTIZ, R., FÉ, C. de la y PONCE, M., 2004. Evaluacion de metodos de almacenaje de semilla de soya (Glycine max. (L.) Merrill) en condiciones de bajos insumos. Cultivos Tropicales [en línea], vol. 25, no. 3, pp. 49-59. [Consulta: 22 noviembre 2019]. ISSN 0258-5936. Disponible en: https://go.gale.com/ps/i.do?p=IFME&sw=w&issn=02585936&v=2.1&it=r&id=GALE%7CA174373711&sid=googleScholar&linkaccess=abs.
PASCUALIDES, A.L. y ATECA, N.S., 2013. Germinación y vigor de morfotipos de semillas de Crotalaria juncea L. (Fabaceae). Phyton [en línea], vol. 82, pp. 313-319. [Consulta: 15 julio 2020]. ISSN 0031 9457. [Consulta: 18 febrero 2020]. Disponible en: https://www.semanticscholar.org/paper/Germinaci%C3%B3n-y-vigor-de-morfotipos-de-semillas-de-L.-Pascualides-Ateca/ccca72612dfc21d522afd0e73e98d6eeede69909.
PRADO, L., SAMANIEGO, C. y UGARTE GUERRA, J., 2010. Estudio de las cadenas de abastecimiento de germoplasma forestal en Ecuador [en línea]. Perú: ASB-World Agroforestry Centre. [Consulta: 27 marzo 2020]. Disponible en: http://old.worldagroforestry.org/downloads/Publications/PDFS/WP16813.pdf.
RIBEIRO, P.R., WILLEMS, L.A.J., MUDDE, E., FERNANDEZ, L.G., CASTRO, R.D. de, LIGTERINK, W. y HILHORST, H.W.M., 2015. Metabolite profiling of the oilseed crop Ricinus communis during early seed imbibition reveals a specific metabolic signature in response to temperature. Industrial Crops and Products [en línea], vol. 67, pp. 305-309. [Consulta: 18 febrero 2020]. ISSN 0926-6690. DOI 10.1016/j.indcrop.2015.01.067. Disponible en: http://www.sciencedirect.com/science/article/pii/S0926669015000692.
RODRÍGUEZ TREJO, D.A. y POMPA GARCÍA, M., 2016. Tamaño, color de nuez y sombra afectan la germinación de Quercus desertícola. Madera y bosques [en línea], vol. 22, no. 2, pp. 67-75. [Consulta: 15 julio 2020]. ISSN 1405-0471. DOI 10.21829/myb.2016.2221325. Disponible en: http://www.scielo.org.mx/scielo.php?script=sci_abstract&pid=S1405 -04712016000200067&lng=es&nrm=iso&tlng=es.
ROSSETTO, C., CONEGLIAN, R.C.C., NAKAGAWA, J., SHIMIZU, M.K. y MARÍN, B., 2000. Germinação de sementes de maracujá-doce (Passiflora alata Dryand.) em função de tratamento pré-germinativo. Revista Brasileira de Sementes, vol. 22, no. 1, pp. 247-252. [Consulta: 27 marzo 2020]. Disponible en: DOI 10.17801/0101-3122/rbs.v22n1p247-252.
RUÍZ PÉREZ, A., ARAMÉNDIZ TATIS, H. y CARDONA AYALA, C., 2017. Efecto del almacenamiento en la calidad fisiológica de semilla de moringa (Moringa oleifera Lam.). Revista U.D.C.A Actualidad & Divulgación Científica [en línea], vol. 20, no. 1, pp. 79-89. [Consulta: 21 enero 2020]. ISSN 2619-2551. DOI 10.31910/rudca.v20.n1.2017.65. Disponible en: https://revistas.udca.edu.co/index.php/ruadc/article/view/65.
RUIZ SÁNCHEZ, M., MUÑOZ HERNÁNDEZ, Y., GUZMÁN, D., VELÁZQUEZ RODRÍGUEZ, R., DÍAZ LÓPEZ, G. S., MARTINEZ, A. Y., & ALMEIDA, F. M. (2018). Efecto del calibre semilla (masa) en la germinación del sorgo. Cultivos Tropicales, 39(4), 51-59. [Consulta: 27 de marzo de 2019]. Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0258-59362018000400007&lng=es&tlng=es.
SAJEEV, N., BAI, B. y BENTSINK, L., 2019. Seeds: A Unique System to Study Translational Regulation. Trends in Plant Science [en línea], vol. 24, no. 6, pp. 487-495. [Consulta: 22 noviembre 2019]. ISSN 1360-1385. DOI 10.1016/j.tplants.2019.03.011. Disponible en: https://research.wur.nl/en/publications/seeds-a-unique-system-to-study-translational-regulation.
ULLOA ULLOA, C. y JORGENSEN, P.M., 1995. Arboles y Arbustos de los Andes del Ecuador [en línea]. Ecuador: Ediciones Abya-Yala. [Consulta: 21 enero 2020]. ISBN 978-9978-04-157-4. Disponible en: https://isbn.cloud/9789978041574/arboles-y-arbustos-de-los-andes-del-ecuador/.
VALVERDE RODRÍGUEZ, K., MORALES, C.O. y GARCÍA, E.G., 2019. Germinación de semillas de Crescentia alata (Bignoniaceae) en distintas condiciones de temperatura, luminosidad y almacenamient. Biología Tropical [en línea], vol. 67, no. 2, pp. 120-131. [Consulta: 27 marzo 2020]. ISSN 0034-7744. Disponible en: https://revistas.ucr.ac.cr/index.php/rbt/article/download/37211/37857/.
VARGAS FIGUEROA, J.A., DUQUE PALACIO, O.L. y TORRES GONZÁLEZ, A.M., 2015. Germinación de semillas de cuatro especies arbóreas del bosque seco tropical del Valle del Cauca, Colombia. Revista de Biología Tropical [en línea], vol. 63, no. 1, pp. 249-261. [Consulta: 18 ferbrero 2020]. ISSN 0034-7744. Disponible en: http://www.scielo.sa.cr/scielo.php?script=sci_abstract&pid=S0034-774420150001 00020&lng=en&nrm=iso&tlng=es.
Conflict of interests:
The authors declare not to have any interest conflicts.
Authors' contribution:
The authors have participated in the writing of the work and analysis of the
documents.
![]()
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0
International license
Copyright (c) 2020 Jorge Luis Cué García, Yajaira Alexandra Cuasque Peñafiel, Hugo Vinicio Vallejos Álvarez, Mario José Añazco Romero, Walter Armando Palacios Cuenca,
Andrés Manolo Carrión Burgos