
Translated from the original in spanish
Influence of substrates and indol butyric acid concentrations on the vegetative propagation of Cinchona officinalis L. (quina) in Amazonia, Peru
Influencia de sustratos y concentraciones de ácido indol butírico en la propagación vegetativa de Cinchona officinalis L. (quina) en Amazonas, Perú
Influência dos substratos e das concentrações de ácido butírico indol na propagação vegetativa de Cinchona officinalis L. (cinchona) na Amazónia, Peru
Tito Sánchez
Santillán1* ![]() https://orcid.org/0000-0002-3352-341X
https://orcid.org/0000-0002-3352-341X
Gelver Silva Valqui2 ![]() https://orcid.org/0000-0002-1471-1810
https://orcid.org/0000-0002-1471-1810
Ariel Kedy Chichipe Puscan1 ![]() https://orcid.org/0000-0003-3298-2961
https://orcid.org/0000-0003-3298-2961
Marcial Trigoso Pinedo1 ![]() https://orcid.org/0000-0001-5007-4049
https://orcid.org/0000-0001-5007-4049
Leidy Gheraldinne Bobadilla
Rivera2 ![]() https://orcid.org/0000-0002-9873-1252
https://orcid.org/0000-0002-9873-1252
Geidy Yecenia Jiménez
Yoplac1 ![]() https://orcid.org/0000-0001-8897-1765
https://orcid.org/0000-0001-8897-1765
1Instituto de Investigaciones de la Amazonia Peruana, Perú.
2Instituto Naciaonal de Innovación Agraria, Perú.
*Correspondence author: titosanchezsantillan@gmail.com
Received: March 5th, 2020.
Approved: April 16th, 2020.
ABSTRACT
The research aims to evaluate the influence of substrates and concentrations of indol butyric acid (AIB) on the vegetative propagation of Cinchona officinalis L. (quina) in Amazonas, Peru. A complete randomized factorial design of 3A x 4B was used, where factor A: substrates (sand, sand 50 % + peat 50 % and peat) and factor B: AIB concentration (0, 1, 2, 3 g.L-1). The study was developed in two phases. In the field, juvenile orthotropic branches of plus trees were collected from the San Jerónimo cloud forest in Peru, located at 2616 meters above sea level. The nursery phase was developed in the experimental center of the National University Toribio Rodriguez of Mendoza-Amazon. The juvenile branches collected from the field were uniformed to 7 cm, leaving two leaves with 50 % of area, were disinfected with Propineb 70 % fungicide 3 g.L-1 of water. It was found that substrates influenced significantly, standing out the sand substrate in the survival and sand + peat showed superiority in rooting, number and length of roots. Concentrations of 2 and 3 g.L-1 of AIB, stimulated root emission and increased the number and size of roots. Substrates and AIB concentrations positively influenced the rooting of young branches of C. officinalis under controlled environmental conditions.
Keywords: Cinchona officinalis; Auxina; Rooting; Juvenile branches; Propagation.
RESUMEN
La investigación tiene por objetivo evaluar la influencia de sustratos y concentraciones de ácido indol butírico (AIB) en la propagación vegetativa de Cinchona officinalis L. (quina) en Amazonas, Perú. Se utilizó un diseño completo al azar con arreglo factorial, de 3A x 4B, dónde factor A: sustratos (arena, arena 50 % + turba 50 % y turba) y factor B: concentración de AIB (0, 1, 2, 3 g.L-1). El trabajo se desarrolló en dos fases, en campo se colectaron ramas ortotrópicas juveniles de árboles plus del bosque de neblina San Jerónimo en Perú, ubicado a 2616 metros sobre el nivel del mar. La fase de vivero, se desarrolló en el centro experimental de la Universidad Nacional Toribio Rodríguez de Mendoza-Amazonas. Las ramas juveniles colectados de campo fueron uniformizados a 7 cm, dejando dos hojas con 50 % de área, se desinfectaron con fungicida Propineb 70 % 3 g.L-1 de agua. Se encontró que los sustratos influyeron significativamente, sobresaliendo el sustrato arena en la sobrevivencia y arena+turba mostró superioridad en el enraizamiento, número y longitud de raíces. Concentraciones de 2 y 3 g.L-1 de AIB, estimularon la emisión radicular e incrementaron el número y tamaño de raíces. Los sustratos y concentraciones de AIB influyeron positivamente en el enraizado de ramas juveniles de C. officinalis en condiciones ambientales controladas.
Palabras clave: Cinchona officinalis; Auxina; Enraizamiento; Ramas juveniles; Propagación.
RESUMO
A investigação visa avaliar a influência dos substratos e concentrações de ácido indol butírico (AIB) na propagação vegetativa de Cinchona officinalis L. (cinchona) no Amazonas, Peru. Foi utilizado um desenho fatorial randomizado completo de 3A x 4B, em que o fator A: substratos (areia, areia 50 % + turfa 50 % e turfa) e o fator B: concentração AIB (0, 1, 2, 3 g.L-1). O trabalho foi desenvolvido em duas fases. No campo, foram recolhidos ramos ortótropos juvenis de mais árvores da floresta nublada de San Jerónimo, no Peru, localizada a 2616 metros acima do nível do mar. A fase de viveiro foi desenvolvida no centro experimental da Universidade Nacional Toribio Rodriguez de Mendoza-Amazónia. Os ramos juvenis recolhidos no campo foram uniformizados até 7 cm, deixando duas folhas com 50 % de área, foram desinfetados com o fungicida Propineb 70 % 3 g.L-1 de água. Verificou-se que os substratos influenciaram significativamente, destacando-se o substrato de areia na sobrevivência e a areia + turfa mostrou superioridade no enraizamento, número e comprimento das raízes. Concentrações de 2 e 3 g.L-1 de AIB, estimulação da emissão radicular e aumento do número e dimensão das raízes. Substratos e concentrações de AIB influenciaram positivamente o enraizamento de ramos jovens de C. officinalis em condições ambientais controladas.
Palavras-chave: Cinchona officinalis; Auxina; Enraizamento; Juvenil; Ramos; Sobrevivência.
INTRODUCTION
Cinchona officinalis L., known as quina tree or cascarilla, belongs to the family Rubiaceae, originating in the South American Andean zone, and distributed from Bolivia, Peru, Ecuador, Colombia and Venezuela (Gómez et al., 2016; Barrutia et al., 2020). It is a medicinal plant, which has been used in treatments of malaria saving many human lives worldwide (Álvarez, 2013).
In Peru, quina is considered a national symbol, and also appears on the national coat of arms (Álvarez, 2013). However, the population is not aware of the level of risk, since with the need to develop agriculture and/or livestock, large quantities of natural forests are deforested, including cinchona plants (Castañeda et al., 2019).
According to media reports, there are 19 species in the genus Chinchona in Peru, which are not yet fully registered in national inventories (RPP, 2017). In the Amazon region, some populations of C. officinalis can be found, mainly in the provinces of Luya (San Jerónimo), Condorcanqui, Bagua, Rodríguez de Mendoza, Bongará (Progreso, Yambrasbamba) (Castañeda et al., 2019), generally located in cloud forests (Carguay-Yaguana et al., 2016).
The main problem in the specie (quina) is propagation, although botanical seed is an alternative, it have reported low germination rates (Buddenhagen et al., 2004). But, there is another faster and simpler method of propagation, asexual propagation using plant tissue (Osuna et al., 2016). Considering that, Conde et al., (2017) obtained successful results by rooting young shoots of quina (higher than 90 %) using auxins.
Based on what has been previous described and in order to find an efficient methodology for mass propagation, the present study aims to evaluate the influence of substrates and concentrations of AIB on the vegetative propagation of C. officinalis L. (cinchona or quina) in Amazonas, Peru.
MATERIALS AND METHODS
Research location
Field phase
The mother plants were selected in the protected area of the cloud forest in the San Jerónimo-Peru district, with coordinates latitude 5° 59' 59,2" South, and longitude 72° 01' 14" West, at an altitude 2 616 m.s.n.m.
In the field, samples of leaves, flowers, fruits and seeds were taken, which were characterized and contrasted dendrometrically following the methodology according to Huamán et al., (2019). The quina samples from San Jerónimo are preserved in the herbarium of the Laboratory of Dendrology at the Toribio Rodríguez National University of Mendoza in Amazonas.
Nursery phase
The rooting process was done in the experimental center of the Toribio Rodríguez National University of Mendoza, in the controlled environments at the Peruvian Amazon Research Institute nursery, located at South latitude 6° 25' 39" and West longitude 77° 32' 13" at altitude 1 714 m.a.s.l.
Selection and collection of plant material
Ten plus trees of 8 to 10 meters in height with a DBH of 25 cm were selected. Juvenile orthotropic branches were cut, with the help of a telescopic scissors. To ensure successful rooting, Denaxa et al., (2012) mentioned to collect a juvenile tissue because its responds better to growth regulators than old and totally differentiated cells. The collected material were wrapped in craft paper, coded and placed in a technopor box, previously moistened to maintain the turgid branches (Vásquez et al., 2018), during the transfer to the experimental center.
Installation of the experiment
In the experimental center, a sub-irrigation chamber was built with dimensions of 3.60 x 1.20 x 0.90 m, length, width and height respectively, an automated irrigation system was conditioned, to regulate the temperature between 18 and 30 °C and relative humidity greater than 80 %. One irrigation per day was programmed for one minute.
In the laboratory, the shoots were disinfected with Propineb 70% PM fungicide, at a concentration of 3 g.L-1 of water, leaving the shoots in the solution for 3 minutes (Inuma et al., 2018).
The disinfected plant material was cut to 7 cm, leaving a pair of leaves cut at 50 % of the leaf area, at the base bevelled cut was made. For the treatment with auxin, it was taken into account, at the time of cutting the base of the sprout, the excess sap that emanated with a paper towel for 60 seconds.
They were placed in AIB solution at different concentrations, diluted in 90° alcohols for 20 seconds (Torres et al., 2014). The shoots were left on paper for 10 minutes at a temperature of 20°C, for the absorption of the auxin. Substrates, 100 % sand, 50 % sand + 50 % peat and 100 % peat were used, disinfected, and placed in 40 x 30 x 10 cm polyethylene trays.
Experimental design
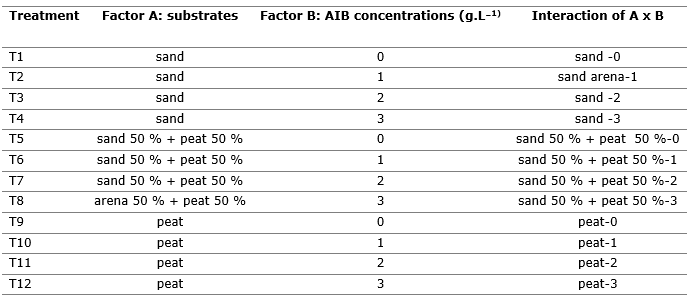
The experiment was set up under a complete randomized design with a factorial arrangement of 3Ax4B, giving 12 treatments, 3 replicates and 36 experimental units (Table 1).
Table 1. - Description of factors and treatments applied to C. officinalis
Evaluation of variables
Survival of juvenile branches: living branches were counted at 60 days, then they were converted to an Excel digital format to transform them into a percentage, dividing the number of living branches, by the total and multiplied by 100.
Percentage of rooting: shoots with the presence of roots, with a size greater than 0,5 cm, were counted (Vásquez, et al., 2018). These were then transformed into a percentage by dividing the total number of rooted branches over the total and multiplying by 100.
Number of roots: roots were counted, with size greater than or equal to 0,5 cm (Vásquez et al., 2018).
Root size: roots were measured with a digital vernier in mm, then transformed to cm, finally they were averaged by repetition.
Statistical analysis
Prior to the analysis of variance, the variables in percentage, survival (Y) and rooting (Y) were transformed with the function T= arcoseno (√ Y). Likewise, the number of roots (Y) was transformed with the logarithm function [T =log10 (Y)], and finally the average values were retransformed into the original units, with the function [Y =100 sin2 (T)], for variables in percent and with the function Y [Y =10T] for the variable number of roots (Muñoz et al., 2009).
ANOVA was performed with a factorial model and multiple Tukey comparison (α=0,05) for survival, and for the variables rooting percentage, number and size of roots, the Friedman non-parametric test and the Wilcoxon Post-Hoc was performed to determine the influence of substrates and AIB concentrations. The statistical software InfoStat version 2019 was used for data processing.
RESULTS AND DISCUSSION
According to the analysis of variance, for the survival of C. officinalis branches evaluated at 60 days, it was observed that there is a significant difference between the substrates and AIB concentrations, acting individually and interactively (Table 2).
Table 2. - Analysis of variance (P< 0.05) for juvenile branch survival
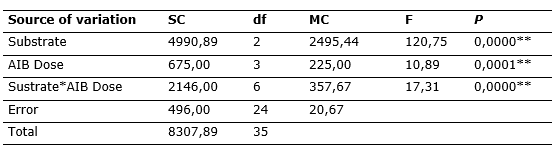
**=significance; SC: sum of squares; df: degrees of freedom; MC: mean squares; F: factor
Table 3. - Tukey test (α=0,05) for the survival of cinchona cuttings, influenced by type of substrates and AIB concentrations
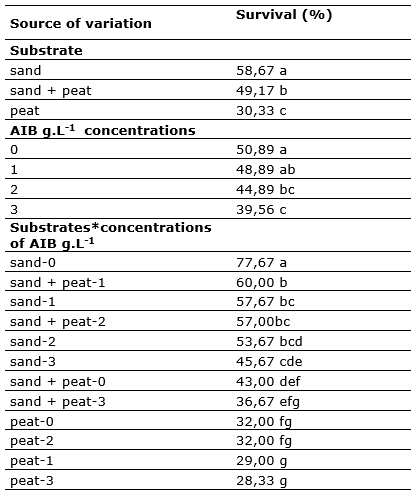
The substrate sand was found to be superior to the others (Table 3), showing a percentage of alive shoots of 58,67 %, the second substrate was the mixture peat + sand with 49,17 %. Likewise, it was found that the substrate peat presented a high mortality exceeding 50 % of treated cuttings. It is known that tender and succulent shoots, most of them die due to rotting, basically influenced by the substrates, since some of them present characteristics such as low porosity, which generate water over-saturation, thus reducing their breathing and creating favorable environments for the proliferation of some pathogens (Mesén, 1998). This statement reinforces what was found regarding the high survival of cuttings in sand (high porosity) and low survival in peat (low porosity).
It was found that the cuttings survived better, when the AIB concentration was lower, so that the cuttings without auxins survived up to 50,89 %; concentrations of 2 and 3 g.L-1 reported higher percentage of mortality, probably due to toxicity (Inuma et al., 2018).
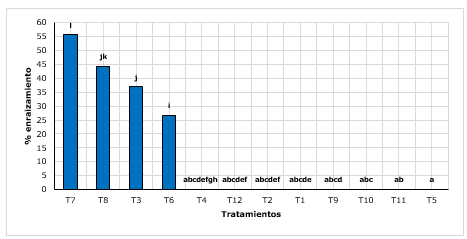
Figure 1. - Wilcoxon Post-Hoc test for rooting percentage according to treatments
Averages
with common lettering do not differ significantly
The T7 treatment (sand + peat with 2 g.L-1 of AIB), presented a rooting of 55,67 %, showing superiority to the T8 (sand + peat with g.L-1 of AIB) with 44.33%. Likewise, most of the other treatments did not emit roots (Figure 1).
Villar et al., (2018), mention that quina is a difficult species to root, due to the content of quinine, an alkaloid that probably inhibits root formation. In another study, Conde et al., (2017) contradict this statement, as they found successful results in rooting quinine through acclimated young shoots in nurseries. They mention up to 88,33 % to 96,67 % of rooting, with and without auxinic treatments, so they explain that they do not necessarily depend on the types of rooting, but on the handling and maintenance given to the shoots during the greenhouse process. Although it is true that the results reported are superior to those found in the present investigation, however, this difference would perhaps be affected by the origin of the plant material, since they worked with cuttings collected directly from the field.
For the rooting of cuttings, it was found that the treatment with a combination of sand and peat showed superior results (55,67 %), compared to the other treatments. Likewise, it was observed that treatments with peat content and low and medium AIB concentrations reported 0 % rooting. Hartmann and Kester (1987), mentioned that the substrates present significant effects in the rooting of plants, varying these according to the vegetal material and the species, highlighting in addition that the best substrate is peat, and if it is combined with sand in different proportions, this one potentiates its activity.
The 2 g.L-1 concentration of AIB stood out in the rooting (55,67 %), followed by the 3 g.L-1 concentration (44,33 %); in this respect, (Hartmann and Kester, 1987; Vásquez, et al., 2018), they mention that there are positive effects of auxins in the rooting, since they stimulate the cell division, increase the transport of carbohydrates and other foliar cofactors to the application sites, as well as the stimulation in the synthesis of DNA in the treated cells. Although, the sensitivity of the cells varies among species, depending on a set of intrinsic factors (Norberto et al., 2001).
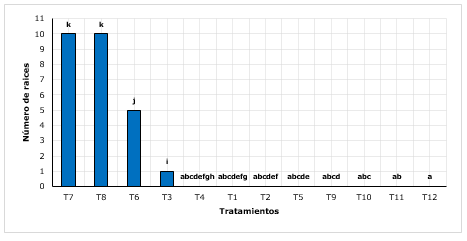
Figure 2. - Wilcoxon post-hoc test for the number of roots according to treatments
Averages with common letters do not differ significantly
For the number of roots, it was found that the T7 treatment (sand + peat with 2 g.L-1 of AIB) and the T8 treatment (sand + peat with 3 g.L-1 of AIB) obtained the highest number of roots (10), higher than the other treatments (Figure 2). Conde et al., (2017), mention when working with young shoots, increased the rooting capacity, so they reported up to 7 roots, on average per shoot. In another study, Castañeda et al., (2019), found a total of 4 roots, which is relatively low compared to what was found, however, these results were found in rooting of cinchona using lignified stakes. Similarly, they reported that the outstanding substrate in root formation was the substrate (35 % leaf litter+35 % sandy loam+20 % organic soil and 10 % sand).
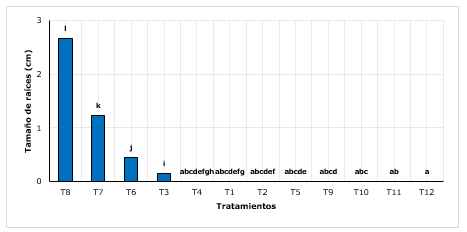
Figure 3. - Wilcoxon Post-Hoc Test for the length of roots according to treatments
Averages with common lettering do not differ significantly
With respect to the length of the roots, it was found that T8 (sand + peat with 3 g.L-1 of AIB) managed to increase on average to a maximum of 2.66 cm, being higher than T7, T6 and other treatments that mostly did not have roots (Figure 3). It is evident that substrates significantly influence root development (Castañeda et al., 2019), roots with sizes up to 4 cm, influenced by the substrate (35 % litter + 35 % sandy loam + 20 % organic soil and 10 % sand).
Another important factor is the plant material which, according to its age, has the capacity to form and develop roots, since the juvenile tissue responds better to growth regulators than old and totally differentiated cells, probably because the areas of the genome that control organ formation are less repressed and can be released with auxin treatments (Denaxa et al., 2012).
On the other hand, Conde et al., (2017) mention that the best results of rooting in quina, were obtained from juvenile sprout which produce roots with lengths 3,23 cm in average, being superior to what was found in this study.
In this way it is also possible to propagate superior trees of the species (Figure 4) associated or not with a tree improvement program and to facilitate species rescue and conservation programs.
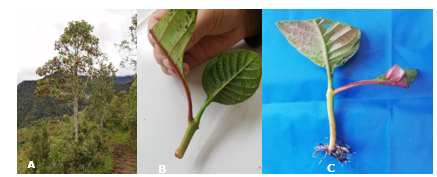
Figure 4. - A- C. officinalis tree plus, in the San Jerónimo-Peru cloud forest; B- callus at 15 days; C- rooted cinchona stakes at 60 days
The vegetative propagation of Cinchona officinalis (quina) was carried out from young orthotropic branches in a sub-irrigation chamber, it presented a high percentage of survival with the substrate sand, however, for rooting, number and length of roots, the outstanding substrate was sand + peat, with concentrations of 2 and 3 g.L-1 of AIB.
REFERENCES
ÁLVAREZ ALONSO, JOSÉ, 2013. El árbol de la calentura. Boletín Instituto Nacional de Salud, [en línea] vol. 19, pp. 214-215. Disponible en: https://repositorio.ins.gob.pe/handle/INS/296
BARRUTIA, R.R.R., BARRETO, I.B. y VELÁSQUEZ, T.D.M., 2020. Germinación de semillas de Cinchona officinalis L. en tres tipos de suelos de Cajamarca, Perú. Revista Cubana de Ciencias Forestales [en línea] vol. 8, no. 1, pp. 75-87. ISSN 2310-3469. Disponible en: http://cfores.upr.edu.cu/index.php/cfores/article/view/488/html
BUDDENHAGEN, C.E., RENTERIA, J.L., GARDENER, M., WILKINSON, S.R., SORIA, M., YÁNEZ, P., TYE, A. y VALLE, R., 2004. The Control of a Highly Invasive Tree Cinchona pubescens in Galapagos1. Weed Technology, [en línea] vol. 18, no. sp1, pp. 1194-1202. ISSN 0890-037X, 1550-2740. DOI 10.1614/0890-037X. Disponible en: https://www.researchgate.net/publication/232663171_The_Control_of_a_Highly_Invasive_Tree_Cinchona_pubescens_in_Galapagos
CARAGUAY YAGUANA, K.A., ERAS GUAMAN, V.H., GONZÁLEZ ZARUMA, D., MORENO SERRANO, J., MINCHALA PATIÑO, J., YAGUANA ARÉVALO, M. y VALAREZO ORTEGA, C., 2016. Potencial reproductivo y análisis de calidad de semillas de Cinchona officinalis L., provenientes de relictos boscosos en la provincia de Loja-Ecuador. Revista Investigaciones Altoandinas, [en línea] vol. 18, no. 3, pp. 271-280. ISSN: 2306-8582. Disponible en: https://dialnet.unirioja.es/servlet/articulo?codigo=5645611
CASTAÑEDA, J.H.V., SABALETA, E.L., HERNÁNDEZ, M.K.Y.B., MEZA, S.N.V. y SÁNCHEZ, L.M.Q., 2019. Comparación de sustratos en la propagación sexual y asexual del árbol de la quina (Cinchona officinalis). Revista de Investigación en Agroproducción Sustentable, [en línea] vol. 2, no. 3, pp. 77-85. ISSN 2520-9760. DOI 10.25127/aps.20183.407. Disponible en: http://revistas.untrm.edu.pe/index.php/INDESDOS/article/view/407
CONDE MONTAÑO, M.E., MORENO SERRANO, J.A., ERAS GUAMÁN, V.H., MINCHALA PATIÑO, J., GONZÁLEZ ZARUMA, D., YAGUANA ARÉVALO, M. y VALAREZO ORTEGA, C., 2017. Multiplicación sexual y asexual de Cinchona officinalis L., con fines de conservación de la especie. Rev. Tzhoecoen [en línea], vol. 9, no. 1. ISSN 1997-3985. Disponible en: http://revistas.uss.edu.pe/index.php/tzh/article/view/463
DENAXA, N. K., S. N. VEMMOS, and P. A. ROUSSOS., 2012. The role of endogenous carbohydrates and seasonal variation in rooting ability of cuttings of an easy and a hard to root olive cultivars (Olea europaea L.). Sci. Hort. [en línea] Vol. 143, pp. 19-28. DOI: 10.1016/j.scienta.2012.05.026 Disponible en: https://www.sciencedirect.com/science/article/abs/pii/S0304423812002579
GÓMEZ SILVERA, A., BERAUN MACEDO, L.A., GÓMEZ RENGIFO, O.J. y LLATAS DUCEP, E., 2016. Procesos de regeneración natural de la quina o cascarilla (Cinchona spp.): en los bosques de neblina del distrito de Kañaris, región Lambayeque. Instituto Nacional de Innovación Agraria (INIA), [en línea] pp. 7. Disponible en: https://repositorio.inia.gob.pe/handle/inia/572
HARTMANN, H.T. y KESTER, D.E., 1987. Propagación de plantas: Principios y prácticas [en línea]. México: Continental. ISBN 978-968-26-0789-9. Disponible en: https://www.worldcat.org/title/propagacion-de-plantas-principios-y-practicas/oclc/503174286?referer=di&ht=edition
Conflict of interests:
The authors declare not to have any interest conflicts.
Authors' contribution:
The authors have participated in the writing of the work and analysis of the documents.
![]()
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International license
Copyright (c) 2020 Tito Sánchez Santillán, Gelver Silva Valqui, Ariel Kedy Chichipe Puscan, Marcial Trigoso Pinedo, Leidy Gheraldinne Bobadilla Rivera, Geidy Yecenia Jiménez Yoplac