
Revista Cubana de Ciencias Forestales. 2020; May-August 8(2):
296-315
Translated from the original in spanish
Nutritional status of Juniperus flaccida Schltdl. and Phoradendron juniperinum Engelm. in response to soil chemical fertilization
Estado nutrimental de Juniperus flaccida Schltdl. y Phoradendron juniperinum Engelm. en respuesta a fertilización química al suelo
Estado nutricional de Juniperus flaccida Schltdl. E Phoradendron juniperinum Engelm. Em resposta à fertilização química do solo
Fanny Libertad
González-Torralva1* ![]() https://orcid.org/0000-0002-0708-8426
https://orcid.org/0000-0002-0708-8426
Miguel Ángel
López-López1 ![]() https://orcid.org/0000-0001-5741-8350
https://orcid.org/0000-0001-5741-8350
Marcos Jiménez-Casas2 ![]() https://orcid.org/0000-0003-0727-017X
https://orcid.org/0000-0003-0727-017X
Dionicio Alvarado-Rosales1![]() http://orcid.org/0000-0001-5941-2446
http://orcid.org/0000-0001-5941-2446
1Universidad Autónoma de Chapingo, Colegio de Postgraduados, México.
2Universidad Nacional Autónoma de México, Colegio de Postgraudados, México.
*Correspondence author: gonzalez.fanny@colpos.mx
Received: February 6 th,2020.
Approved: May 28 th, 2020.
ABSTRACT
Juniperus flaccida Schltdl. is a very important forest species which is severely affected by Phoradendron juniperinum Engelm. at El Cardonal, Hidalgo, Mexico. The objective of this research was to evaluate the response, in terms of tree twig biomass and nutrient status of both the tree and its parasitic species, to fertilization with nitrogen (N), phosphorus (P), and potassium (K), in a natural forest of J. flaccida. The fertilizer rates and sources were 0,1000; 0, 300 and 0, 450, 900 g tree-1 of urea, phosphoric acid and potassium sulphate, respectively. The experimental design was a completely randomized factorial (2x2x3) with six replicates. Soil analysis showed low levels of N and P, and adequate levels of K in the site. Nitrogen or phosphorus alone did not significantly affect twig biomass, despite of being deficient nutrients in the study site. J. flaccida displayed higher concentrations of N, Mg, Cu, Mn, and B than P. juniperinum and the latter, showed higher concentrations of P, K, Ca, Fe, and Zn than its host species. Application of nitrogen significantly decreased the concentration of K in P. juniperinum and influenced the partition of foliar P between J. flaccida and P. juniperinum, in favor of the host tree and to the detriment of the parasitic species. Application of K increased foliar N in J. flaccida. Vector analysis showed that the combination of phosphorus with the highest dose of potassium led to an increase in twig biomass of J. flaccida.
Keywords: Soil analysis; Vector analysis; Fertilizers; Parasitic plant.
RESUMEN
Juniperus flaccida Schltdl. es una especie forestal muy importante que, en El Cardonal, Hidalgo, México es fuertemente atacada por Phoradendron juniperinum Engelm. El objetivo de esta investigación fue evaluar la respuesta de ramillas de J. fláccida en cuanto a su biomasa,su estado nutrimental y el de su planta parásita, con relación a las aplicaciones de nitrógeno (N), fósforo (P) y potasio (K), en un bosque natural de Juniperus flaccida. Las dosis y fuentes de fertilizantes fueron 0 y 1000; 0 y 300; y 0, 450 y 900 g árbol-1 de urea; ácido fosfórico y sulfato de potasio, respectivamente. El diseño experimental utilizado fue completamente al azar con arreglo factorial 2x2x3, con seis repeticiones. Los análisis de suelo mostraron bajos niveles de N y P, y suficientes de K en el sitio. Las aplicaciones individuales de N y P no tuvieron efectos significativos en biomasa, a pesar de ser deficientes en el área experimental. J. flaccida presentó mayores concentraciones foliares de N, Mg, Cu, Mn y B que P. juniperinum, y éste presentó mayores concentraciones de P, K, Ca, Fe y Zn que su hospedero. La aplicación de nitrógeno disminuyó significativamente la concentración de K en P. juniperinum e influyó en la partición de P foliar entre J. flaccida y P. juniperinum, en favor de la hospedera y en detrimento de la parásita. El aporte de K incrementó la concentración de N del follaje de J. flaccida. Los análisis de vectores mostraron que la combinación de fósforo con la dosis alta de potasio propició un incremento en biomasa de J. flaccida.
Palabras clave: Análisis de suelos; Análisis de vectores; Fertilizantes; Planta parásita.
RESUMO
Juniperus flaccida Schltdl. É uma espécie florestal muito importante que, em El Cardonal, Hidalgo, México, é fortemente atacada por Phoradendron juniperinum Engelm. O objectivo desta investigação foi avaliar a resposta dos ramais de J. flaccida em termos da sua biomassa, do seu estado nutricional e do da sua planta parasita, em relação às aplicações de nitrogénio (N), fósforo (P) e potássio (K), numa floresta natural de Juniperus flaccida. As doses e fontes de fertilizantes foram 0 e 1000; 0 e 300; e 0, 450 e 900 g de ureia de árvore 1; ácido fosfórico e sulfato de potássio, respectivamente. O desenho experimental utilizado foi completamente aleatório num arranjo fatorial 2X2X3, com seis réplicas. As análises do solo mostraram níveis baixos de N e P, e K suficiente no local. As aplicações individuais de N e P não tiveram um efeito significativo na biomassa, apesar de serem deficientes na área experimental. J. flaccida apresentou concentrações foliares de N, Mg, Cu, Mn e B mais elevadas do que P. juniperinum, e este último apresentou concentrações mais elevadas de P, K, Ca, Fe e Zn do que o seu hospedeiro. A aplicação de azoto diminuiu significativamente a concentração de K em P. juniperinum e influenciou a divisão do foliar P entre J. flaccida e P. juniperinum, em favor do hospedeiro e em detrimento do parasita. A contribuição de K aumentou a concentração de N da folhagem de J. flaccida. As análises vectoriais mostraram que a combinação do fósforo com a elevada dose de potássio levou a um aumento da biomassa de J. flaccida.
Palavras-chave: Análise dos solos; Análise de vetores; Fertilizantes; Planta parasita.
INTRODUCTION
Biotic disturbances affect a wide range of tree species in all climates and contribute to increasing global tree mortality rates (Szmidla et al., 2019). Mistletoe belongs to a large group of parasitic plants that establish long-lasting relationships with a wide range of host tree species. With climate change, ecophysiological stress is increasing, which may make trees more susceptible to mistletoe infection and may even lead to higher mortality rates in forest stands (Griebel et al., 2017).
Phoradendron juniperinun (muérgado del táscaste) is currently parasitizing Juniperus flaccida (táscate) in the region of El Cardonal, in the State of Hidalgo, Mexico; according to the literature there are no records of distribution of this mistletoe species in the study area (Geils et al., 2002), which means that it is spreading by finding suitable conditions for its establishment. This interaction between P. juniperinum and J. flaccida is causing serious disturbance to the natural forest of J. flaccida in the region. Among the most important physiological interactions between host plants and plants are nutrient and water relationships (Glatzel and Geils 2009). Despite the importance of such relationships between host trees and mistletoes, relatively little is known, especially regarding nutritional requirements and their supply to parasite plants (Okubamichael et al., 2011; Raya-Pérez et al., 2014).
Velasco (1999), mentions that carrying out nutritional management of trees through chemical fertilization, represents an important activity in the control of pests and diseases, as well as an integral component in productivity whether in the agricultural or forestry area. When trees are established in poor or nutrient deficient soils, they will respond by showing poor growth and development, as well as susceptibility to pathogen attack. According to Reyes-Pozo et al. (2018), plants whose nutritional conditions are balanced become more tolerant or less susceptible to the attack of pests and/or diseases, since they can protect themselves from possible infections and/or limit those present.
The nutritional status and vigor of mistletoes depends largely on the nutritional status of the hosts from which they absorb nutrients, so a thorough understanding of parasite-host nutritional relationships can lead to possible nutritional management strategies for trees to decrease their susceptibility to attack by parasitic plants. In this context, the present study aimed to evaluate the nutritional response of J. flaccida and P. juniperinum, its parasitic plant in the region, to chemical fertilization of the host with N, P and K individually and in combination.
MATERIALS AND METHODS
The experiment was conducted from August 2018 to September 2019, in the town of San Miguel Tlazintla, El Cardonal, Hidalgo, Mexico, in a natural forest of Juniperus flaccida Schltdl. The site is located at coordinates 18° 42' 20.2" north latitude and 90° 44' 09.0" west longitude and at an altitude of 2 094 m. The site has a temperature range between 12 - 22°C and an average rainfall of 430 mm. It has a semi-dry temperate climate with summer rains; the dominant soil is leptosol (INEGI 2009) (Figure 1).
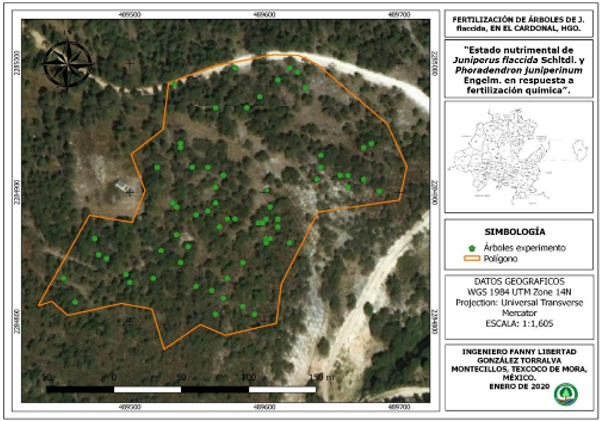
Figure 1. - Study Area; Bienes Comunales San Miguel Tlazintla, El Cardonal, Hidalgo, Mexico
Experimental and treatment design
A 2x2x3 factorial experiment was performed, with six repetitions, taking a tree as the experimental unit. Table 1 shows the factors (nutrients) of variation that were tested, such as, nitrogen (N), phosphorus (P) and potassium (K). The factors studied were defined based on a previously conducted chemical soil analysis, while the levels (fertilization rates) of each was determined based on the published results of a fertilization trial on a 12-year-old plantation of Cupressus lusitanica in Colombia (Tschinkel, 1972).
Table 1. - Variation factors of the fertilization factorial experiment established in the Ejido of San Miguel Tlazintla, Municipality of El Cardonal, Hidalgo
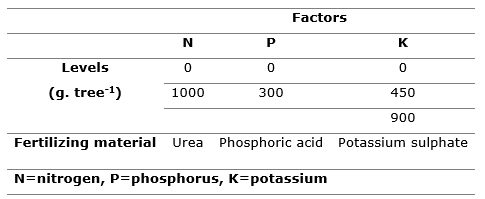
The combination of the levels of the factors studied produced 12 treatments mentioned below, whose values in brackets correspond to the quantities applied (g.tree-1) of urea, phosphoric acid and potassium sulphate, in that order: T1 (0,0,0); T2 (0,0,450); T3 (0,0,900); T4 (0,300). 0); T5 (0,300,450); T6 (0,300,900); T7 (1000,0,0); T8 (1000,0,450); T9 (1000,0,900); T10 (1000,300,0); T11 (1000,300,450) and T12 (1000,300,900).
Fertilization
The application of treatments was carried out starting with the selection of the experimental units, trying to make them homogeneous in terms of diameter, in order to avoid effects due to differences in size. In addition, we tried to ensure that all the experimental units had the same degree of mistletoe incidence. Once the trees were selected, the diameter at breast height (DBH) of each individual was measured with the help of a tape measure, the fertilization treatment was applied according to a previously performed randomization and the trees were labeled. The DBH was measured only to ensure uniformity of the dimensions of the experimental trees.
The fertilizers were applied in granulated form for the case of nitrogen (urea) and potassium (potassium sulfate), in the case of phosphorus (phosphoric acid) it was applied in liquid form mixing the dose determined in five liters of water and pouring the solution over the dripping area, with a watering can.
Variables evaluated
The variables evaluated were: soil fertility (chemical analysis), water potential for both species; growth variable (increase in biomass of branches of J. flaccida) and physiological variables (nutritional concentration in the foliage of J. flaccida and P. juniperinum).
The water potential was taken with the help of a Scholander pressure chamber, model 3005 Soil Moisture. For this purpose, four individuals of J. flaccida were selected and a branch was selected in each of them. Within each selected branch, a mature and biotic free branch was collected, close to a P. juniperinum plant, from which a branch was also collected. The cut of the samples was made in a uniform way collecting a bunch of approximately 10 cm; later this was introduced in the pressure chamber, opening the gas valve and taking the measurement at the moment that a bubble of sap emerged from the base of the bunch.
Obtaining biomass from twigs was taken by removing, with the use of a telescopic scissor, five twigs from the last growth flow. These were distinguished by their light coloring. The samples were taken to the laboratory where they were dried in a FELISA drying oven at 70ºc for 72 hours. Once the samples were dry, they were weighed on an analytical balance, thus obtaining the biomass of the branches of each of the trees belonging to the fertilization experiment.
The initial nutritional status was determined by collecting foliage of both species from nine randomly selected trees within the experimental area, following the protocol of Wells and Allen (1985). The samples were processed and sent to the Plant Nutrition Laboratory of the Postgraduate School, to determine the foliar concentrations of N, P, K, Ca, Mg, Fe, Cu, Zn, Mn and B.
Twelve months after the fertilization experiment was established, foliage samples were taken from three individuals per treatment, which were obtained from the upper third of the crown. These samples were analyzed for the determination of N, P and K, in order to know the response of the trees to the application of the treatments.
During the soil sampling carried out before the application of the treatments, eight samples were taken at random within the study area, at a depth of 30 cm. From these samples, four composite samples were formed, which were taken to the same laboratory as the leaf samples, for the determination of pH, MO, CIC, N, P, K Ca, Mg, Zn, Fe, Mn and Cu. The soil results obtained were interpreted with the help of NOM-021 SEMARNAT 2001 and were the basis for deciding the nutrients to be tested in the experiment.
Methods of chemical analysis
The determination of P and K concentrations was performed on the extract resulting from the wet digestion of dry plant material with sulfuric acid, perchloric acid and hydrogen peroxide (1.3:0.7:1, v:v:v), as described by Alcantar and Sandoval (1999). The extracts were read on a plasma induction and emission spectrometry machine (Agilent 725 Series ICP-OES).
The N concentration was determined in a 10 mL aliquot of the digestate described above, by distillation of the sample and titration with sulfuric acid.
Statistical analysis
With the help of the SAS statistical package (version 9.0), the statistical analysis consisting of an Analysis of Variance (ANAVA) and a Tukey mean comparison test (á=0.05) was carried out for the effects of treatments on the concentration of N, P and K in the foliage of J. flaccida and P. juniperinum after fertilization treatments. Branch biomass, along with concentrations and nutrient contents were analyzed using the vector graphic analysis technique (Timmer and Stone, 1978). Using regression analysis, the dependence of leaf concentrations of P. juniperinum on those of Juniperus flaccida was examined. The water potentials of J. flaccida and P. juniperinum were compared using a T-test.
RESULTS AND DISCUSSION
Preliminary soil and leaf analysis
According to Table 2, the soils of the study site, interpreted in accordance with the Mexican Official Standard 021 of SEMARNAT (Ministry of Environment and Natural Resources 2002) only present low levels of nitrogen and phosphorus, while the other properties analyzed are at adequate or slightly high levels.
Table 2. - Chemical properties of the soil in the Juniperus flaccida forest of the Ejido of San Miguel Tlazintla, El Cardonal, Hidalgo
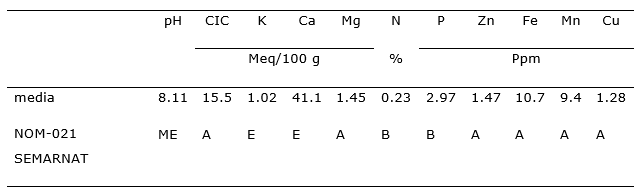
ME: Moderate Alkaline; A: Adequate; B: Low; E: High.
The results of the preliminary leaf analysis (before the application of the treatments), regarding the macro-elements (N, P, K, Ca and Mg) are shown in Figure 2; while those regarding the concentrations of the micro-elements (Fe, Cu, Zn, Mn and B) are presented in Figure 3.
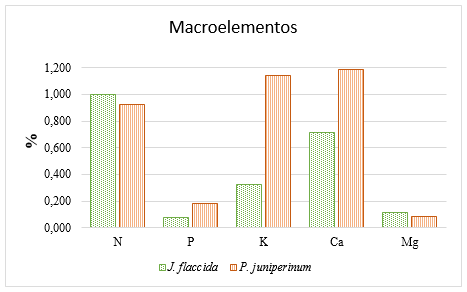
Figure 2. - N, P, K, Ca and Mg concentrations in the foliage of J. flaccida and P. juniperinum, before application of fertilization treatments
The Figure 2 shows that mistletoes have high concentrations of P (0.182 %), K (1.146 %) and Ca (1188 %), compared with the corresponding concentrations in J. flaccida. These results are consistent with those obtained by Raya-Pérez et al., 2014, who observed that mistletoes (Psittacanthus calyculatus) accumulated high levels of potassium, a nutrient that in the present investigation is 253 % above the concentration presented by the host (J. flaccida). On the other hand, Glatzel (1983), investigated the concentrations of other nutrients and observed that N was the only nutrient not accumulated by mistletoes at higher levels than their hosts, coinciding with what was obtained in the analyses reflected in Figure 2, where J. flaccida presents a foliar concentration of N that is 8.78 % higher than the concentration of this nutrient in P. juniperinum. As in the case of N, in the present study, the concentration of Mg in the host was 34 % higher than the concentration of the same nutrient in the host plant.
The analyses obtained in the present investigation agree with a study carried out by Panvini and Eickmeier (1993), who obtained the mineral concentrations of Phoradendron leucarpum and several host species, finding that mistletoe had nutrient concentrations from 0.97 to 2.88 times higher than its host trees. In that study the data collected showed higher concentrations of K, N, Ca, Mg, P, Na, Fe, Zn and Cu in the host.
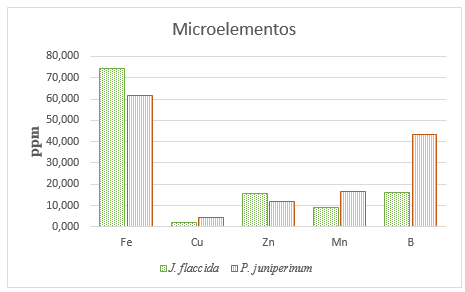
Figure 3. - Concentrations of Fe, Cu, Zn, Mn and B in the foliage of J. flaccida and P. juniperinum, before the application of fertilization treatments
The Figure 3 shows the concentrations of microelements and shows higher concentrations of Cu (4.47 ppm), Mn (16.81 ppm) and B (43.35 ppm), in P. juniperinum compared to those found in J. flaccida (1.98, 9.11 and 16.11 ppm, respectively). These microelements are found 66, 46 and 63 % in lower concentration in the host species than in the parasite plant.
It is very likely that the higher concentrations of most nutrients found in mistletoe, relative to those in the host, are an adaptation related to the host's need to absorb water from the host (Raftoyannis et al., 2015); i.e., the host plant (mistletoe) needs to absorb water from the xylem of the host tree. Since water always moves from higher water potentials to sites with lower water potentials, in order for the mistletoe to take up water from the host tree, the water potentials of the mistletoe need to be lower (more negative) than those of the host. The high concentrations of salts in mistletoe tissues contribute to this condition, generating a flow of water from the tree (in this case J. flaccida), to the mistletoe (P. juniperinum).
Determinations of water potentials (Ψ) in host tree and plant branches indicated that the average Ψ in P. juniperinum (-36.12 bar) is significantly lower (Pr>0.009; Table 3) than that of J. flaccida (Ψ=-29.75 bar). These results corroborate what has been obtained in other research where it has been shown that mistletoes maintain higher transpiration rates and are less efficient in water use than their hosts (Garkoti et al., 2002). Hosseini et al., (2007), observed that Viscum album, a parasitic species of Carpinus betulus, maintains a higher transpiration rate in order to achieve mainly nitrogen absorption. On the other hand, Glatzel and Geils (2009), mentioned that mistletoes maintain a more negative water potential than their host species, in order to avoid stomatal closure and withering, which may explain the results obtained in the present research, which corroborates the low water potential that P. juniperinum maintains with respect to J. flaccida.
Table 3. - Test of t for the water potential of
Juniperus flaccida and Phoradendron
juniperinum
in the Ejido San Miguel Tlazintla, El Cardonal, Hidalgo, Mexico
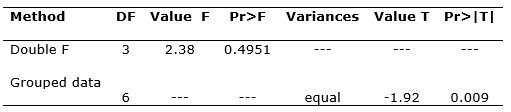
DF: Degree of freedom
Nutritional status of J. flaccida and
P. juniperinum
The Figures 4 a, b and c present the leaf concentrations of N, P and K, respectively for the species of interest. In the case of nitrogen (Figure 4a), in all treatments J. flaccida presented the highest concentrations compared to P. juniperinum, highlighting the T6 (P 300-K 900) and T12 (N 1000-P 300-K 900) treatments, which presented the highest concentrations of this nutrient in J. flaccida.
According to Figure 4a, in all treatments, including those that did not contemplate nitrogen application, the leaf concentration of this nutrient in the host is, on average, 18% below the concentration of the same nutrient in the host tree. These results are consistent with those obtained by Bowie and Ward (2004), who determined that N is the only nutrient found in lower concentrations in mistletoe (65 %), a condition that is consistent with the theory of passive nutrient absorption.
The results obtained in this research differ from those obtained by Türe et al., (2010), since the analyses carried out by these authors showed that nitrogen was stored in a higher concentration in the mistletoe Viscum album than in the host species under observation. This means that the proportions between N concentrations in host plants and hosts can be characteristics associated with the very combination of host species and hosts.
Panvini and Eickmeier (1993) suggest that N is a nutrient that limits the growth of mistletoe and that the mechanism they use to access this nutrient is to maintain high transpiration rates. This coincides with the results of the present study, which show that mistletoes maintain lower water potentials than their host species.
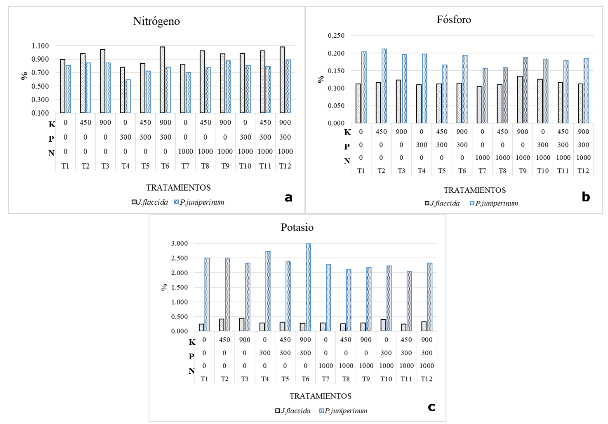
Figure 4. - Foliar concentration of N, P and K in
J. flaccida and P. juniperinum,
in a
fertilization experiment in Ejido San Miguel Tlazintla, municipality of El Cardonal, Hidalgo
Figure 4b shows the phosphorus concentrations for the twelve treatments in both species. J. flaccida presents on average, 33% lower phosphorus concentration than P. juniperinum. Within the treatments analyzed, the second (450 g of K) and control treatments stand out, both of which present a higher concentration of P, even though this nutrient was not supplied to the trees in those treatments. The application of P to the trees did not show increases in the concentrations of this nutrient in the foliage of either species (J. flaccida or P. juniperinum; Figure 4b), probably due to the fact that the applied P was converted into calcium phosphate, as a consequence of the high alkalinity conditions of the site (Hopkins and Ellsworth 2005); however, Figure 4b clearly shows that the application of N did affect the distribution of P between host and host, noting that the application of 1000 g of urea per tree favored the accumulation of P in the host, to the detriment of the foliar P of the parasite plant (Table 4).
There are no references in the literature related to the above effect; however, this finding suggests that the application of N in infested areas similar to the site studied could lead to improved P partitioning between host species and host in favor of the former, which could ultimately improve tree vigor, especially in P-deficient sites. The results of the present research are consistent with those obtained by authors such as Glatzel (1983), Panvini and Eickmeier (1993), Bowie and Ward (2004) and Türe et al, (2010), who found that P, is one of the nutrients that is stored in greater concentration in mistletoe tissue compared to the host species analyzed.
Table 4. - Effects of nitrogen application on leaf N, P and K partitioning between Juniperus flaccida and Phoradendron juniperinum
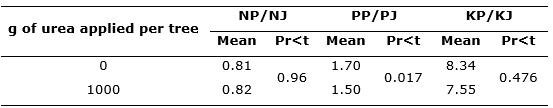
NP: Foliar concentration of N in P.
juniperinum; NJ: Leaf concentration of N in
Junuperus flaccida; PP: Foliar concentration of P in
P. juniperinum; PJ:
Foliar concentration of P in
J. flaccida; KP: Foliar concentration of K in
P. juniperinum; KJ: Foliar concentration of K in
J. flaccida.
Potassium (Figure 4c) is one of the main nutrients in mistletoe invaded tree scenarios, which is stored in very high concentrations, within the parasite plant (Glatzel 1983; Türe et al., 2010 and Tennakoon et al., 2011). The authors mentioned above maintain that most plant species have a preference for the absorption of this nutrient, since it plays a role as a carrier of other elements, in addition to being essential for osmoregulation and stomata control.
The analysis of variance in Table 5 shows the effects of fertilization treatments on the concentrations of nitrogen, phosphorus and potassium in the foliage of J. flaccida and P. juniperinum.
Analysis of variance indicates that leaf concentrations of N, P and K were affected by the treatments (Table 5). It is observed that N had significant effects on phosphorus and potassium concentrations in the foliage of P. juniperinum (Pr = 0.03 and Pr = 0.01, respectively). On the other hand, K, influenced the nitrogen concentration in the foliage of J. flaccida (Pr=0.01).
In the case of interactions between nutrients, no significant effects on the concentrations of N, P and K in the foliage of any of the species were found. However, the interaction of N*P stands out, which had a slight influence on the concentrations of N in J. flaccida (Pr=0.07) and P in P. juniperinum (Pr=0.07; Table 5).
Table 5. - Significance, according to the analysis of variance, of the effects of fertilization
treatments on the concentrations of N,
P and K in the foliage of
J. flaccida and P. juniperinum, in the Ejido
of San Miguel Tlazintla, Municipality of El Cardonal, Hidalgo
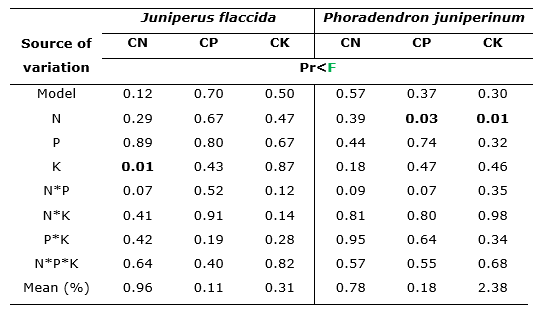
Pr<F (α=0.05); N: Nitrogen; P: Phosphorus; K: Potassium; CN: Foliar
nitrogen concentration;
CP: Foliar phosphorus concentration; CK: Foliar potassium concentration.
Comparison of Tukey's means (α = 0.05, Table 6), shows that phosphorus application had no significant effect on N, P and K concentrations in the foliage of either study species; however, when N was applied to the trees, a lower mean potassium concentration in the foliage of P. juniperinum was presented, suggesting that this practice may contribute to reduce mistletoe vigor, by decreasing the concentration of solutes in the individual, hindering the flow of water and ions from the host tree. On the other hand, it is observed that with the application of 900 g of K, there was a significant increase in the foliar concentration of N in the J. flaccida foliage (Table 6). This effect could have implications on the nutritional management decisions of J. flaccida forests affected by mistletoes, since the supply of K to the host tree improves the nutrition of N, while the application of N decreases the uptake of K by the parasite plant.
Table 6. - Tukey test (α=0.05) for N, P and K concentrations in the foliage of J. flaccida and P. juniperinum, after fertilization treatments
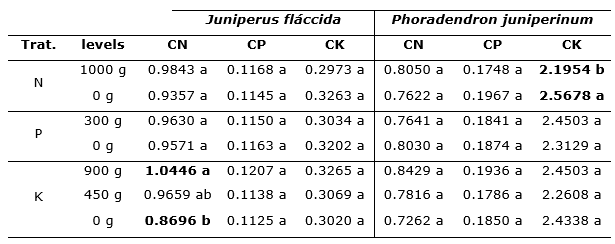
N: Nitrogen; P: Phosphorus; K: Potassium; CN: Foliar nitrogen concentration; CP: Foliar
phosphorus concentration; CK:
Foliar potassium concentration; within a treatment and a foliar
nutrient, same letters are not significantly different
(α=0.05).
The Figure 5 (a, b and c) shows the behavior of the increase in biomass, concentration and content of N, P and K in the foliage of J. flaccida, with respect to fertilization treatments.
The P*K2 treatment (300 g P * 900 g K per tree) presented the highest growth in terms of biomass of five branches of J. flaccida, compared to the rest of the treatments (Figure 5). This coincides with the diameter data obtained for the trees that received this treatment, which presented greater increases in diameter or avoided the decrease of this variable than in other treatments that caused the drought that occurred in the area of study during the experimental year (2018).
In the case of N (Figure 5a), it can be seen that three of the twelve treatments (P*K1, N and P) present lower nitrogen concentrations than the critical concentration (0.826 %) generated for J. flaccida (González-Torralva 2020). The lack of response of the other treatments was perhaps due to some other limiting factor, since some treatments such as N*P*K2 (1000 g N, 300 g P and 900 g K), N*K1 (1000 g N and 450 g K), K2 (900 g K), N*P*K1 (1000 g N, 300 g P and 450 g K) and N*P (1000 g N and 300 g P), generated high concentrations of N, but no response was obtained from the increase in branch biomass. This could be due to the drought condition to which the experimental area was subjected, or to some other limiting factor. As for the treatments where N*K was combined, the low response could perhaps be due to the antagonism that exists between these nutrients (Mengel and Kirkby 1982).
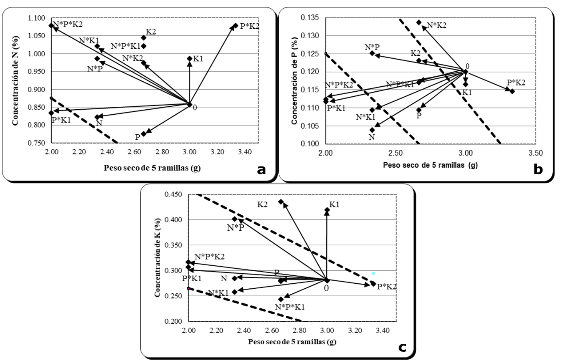
Figure 5. - Effect of treatments on the biomass of five branches, concentration and
nutrient content
in an experiment of fertilization with N, P and K in a natural forest of
J. flaccida, in El Cardonal, Hidalgo
As for P, soil analysis indicated a deficiency of this element. According to this, adding phosphorus would be expected to increase tree growth; however, this did not happen significantly. In Figure 5b, it is observed that the P*K2 treatment (300 g P * 900 g K per tree), presented a considerable increase in branch biomass (3.33 g), compared to the rest of the treatments, which presented biomass even below the control treatment, as well as a decrease in phosphorus uptake, even when this nutrient was applied. A possible explanation for this behavior is that the soil within the study area presents high levels of calcium, which when phosphorus is applied, contributes to the formation of calcium phosphates, which makes the phosphorus unavailable to the plant (Mengel and Kirkby 1982). This treatment (P*K2) presents a concentration of P (0.115 %) very close to the critical concentration (0.11 %) reported for this species (González-Torralva, 2020).
With regard to potassium, soil analysis in the study area indicates that there is a high level of this nutrient, so it would be expected that when K is applied, there would be no response from individuals in terms of growth. In Figure 5c, it can be seen that the best treatment in terms of increase in biomass of five branches is P*K2 (300 g P * 900 g K), but it resulted low in K concentration, since it presents 0.274 % of K in the foliage of J. flaccida, a concentration lower than the critical one reported for this species (0.301 %; González-Torralva, 2020). In the case of the K1 and K2 treatments, (450 and 900 g of K, respectively), their increase in biomass was even below that of the control treatment; especially the K2 treatment, but the K concentration for both treatments was almost 0.100 % above the critical concentration of the species of interest. These high concentrations of K in the foliage and low growth are consistent with the diagnosis of K sufficiency at the site (soil analysis), but are contrary to the higher growth in diameter, recorded in the trees that received K, in which case, it is likely that the K supply contributed to the conservation of the turgidity of the tree stem cells, thus avoiding the reduction in diameter experienced by the trees that did not receive this treatment. Vázquez-Cisneros et al., (2018) reported that an experimental plantation of Pinus greggii, growing in soil with sufficient potassium, showed positive growth responses when this nutrient was applied, concluding that potassium possibly improved cell turgidity in the plants and the incorporation of carbon.
By doing an integration of the results of soil, foliage and tree growth analysis it can be stated that on the site, despite the fact that the soil analysis indicates high levels of potassium, the best diameters were obtained when applying this nutrient.
Functional relationships between foliar concentrations of N, P and K between J. flaccida and P. juniperinum
The Figure 6 (a, b and c) shows the behavior of the foliar concentration of nutrients in
Phoradendron juniperinum, as a function of the corresponding nutrient concentrations
in Juniperus flaccida.
The relationship between foliar N in P. juniperinum and the corresponding N in J. flaccida (Figure 6a) is described by a saturation curve (R2=0.77) which indicates that when the foliar N concentration in J. flaccida is less than 0.975 %, the concentration of the same nutrient in the P. juniperinum increases as that of J flaccida.; however, when the N concentration in the host tree reaches the mentioned N concentration, the parasite plant becomes unable to accumulate more nitrogen in its foliage. This behavior may be relevant from the point of view of nutritional management and control of the parasitic plant, since it suggests that N application is a practice that can help manage the status of this nutrient in the tree, even in the presence of the parasitic plant, with the additional effect of lowering the concentration of K in the mistletoe (Table 6). The R2 value indicates that 77 % of the nitrogen concentration present in mistletoe foliage depends on the nutrient concentration of the host. This explains that when there is little nitrogen in the tissues of J. flaccida, as the concentration of N in this species increases, it also increases in the mistletoe, but when the host has abundant N, this nutrient probably becomes sufficient for the parasite plant and the concentrations of nitrogen in the plant become more variable, possibly because other factors limiting the growth of the plant appear.
In the case of P (Figure 6b), the behavior is similar to that of nitrogen, only in this case, the model that best represented the relationship between tree leaf P and mistletoe was of the linear type, with an R2 of 0.1106. That is, by increasing the host P leaf concentration, the concentration of the nutrient in the mistletoe increases, although in this case, the leaf concentration of P in J. flaccida only explains 11 % of the P concentration in the mistletoe. This means that P uptake by mistletoe is influenced by a larger number of factors and is more complex than in the case of N.
Figure 6c shows that when K concentrations in the foliage of J.flaccida. are low, in the mistletoe they can be low or high, but when such host concentrations are high, apparently in the mistletoe they are low; if this is consistent, then it indicates that in this region of the state of Hidalgo, the application of K to the soil would raise the concentrations of this nutrient in J. flaccida. If this is consistent, then it indicates that in this region of Hidalgo state, application of K to the soil would raise the concentrations of this nutrient in J. flaccida without generating major increases in the concentration of the same in the mistletoe, which could contribute to increasing the differentials of water potentials between the host and the parasite, in favor of the former (lower potentials in J. flaccida than in P. juniperinum), which could hinder the absorption of water and other nutrients by the mistletoe and promote its weakening.
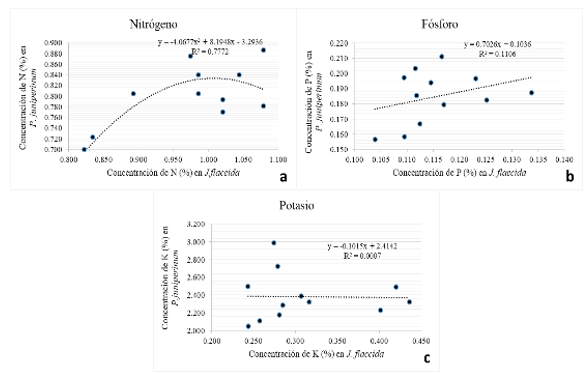
Figure 6. - Behavior of nutrient concentration (N, P and K) in P. juniperinum as a function of corresponding concentrations in J. flaccida
It was previously mentioned that mistletoe has very high K requirements, so by decreasing the concentration of this nutrient in the parasite plant tissue, a nutritional imbalance would be generated, which could decrease the mistletoe's vigor and increase the trees' vigor. In the present study, this behavior was reflected in the fact that the trees that received K showed an increase in diameter or at least a decrease was avoided due to drought conditions, as occurred in trees that did not receive this nutrient.
P, K, Ca, Fe and Zn show higher concentrations in mistletoe foliage than in J. flaccida, the opposite is true for N and Mg, Cu, Mn and B.
The water potential is lower in leaf of P. juniperinum than in J. flaccida branches located near the base of the mistletoe.
The application of N influences the partition of P foliar between J. flaccida and P. juniperinum, favoring of the host species and to the detriment of the parasite.
The application of N significantly decreases the foliar concentration of K in the parasitic plant, while the supply of K increases the concentration of N in J. flaccida.
The correlation between the foliar concentration of N in J. flaccida and the corresponding concentration in P. juniperinum is lost when N concentrations in J. flaccida are high.
There is no correlation between the foliar concentration of K in J. flaccida and the corresponding in P. juniperinum.
The combination of P with the high dose of K is the only treatment that promotes the increase in branch biomass in J. flaccida in the study area.
ACKNOWLEDGEMENTS
To the National Council of Science and Technology (CONACYT), for the scholarship granted to the first author for the development of his Master of Science studies.
To the Forestry Technician, Engineer Eduardo Vargas Solís and to the Ejidal Commissariat C. Alfredo Isidro Cervantes, for having allowed the research to be carried out on their ejido lands.
REFERENCES
ALCÁNTAR, G. G. y SANDOVAL, M. V. 1999. Manual de análisis químico de tejido vegetal. Guía de muestreo, preparación, análisis e interpretación. Publicación especial No. 10 de la Sociedad Mexicana de la Ciencia del Suelo A. C. Chapingo, México. 156 p.
BOWIE, M., y WARD, D. 2004. Water and nutrient status of the mistletoe Plicosepalus acaciae parasitic on isolated Negev Desert populations of Acacia raddiana differing in level of mortality. Journal of Arid Environments, 56(3), 487508. Recuperado de: https://doi.org/10.1016/S0140-1963(03)00067-3
GARKOTI, S.C., AKOIJAM, S.B. y SINGH. S.P. 2002. Ecology of water relations between mistletoe (Taxillus vestitus) y its host oak (Quercus floribunda). Tropical Ecology 43: 243-249. Recuperado de: http://docplayer.net/35453400-Ecology-of-water-relations-between-mistletoe-taxillus-vestitus-and -its-host-oak-quercus-floribunda.html
GEILS, B. W.; CIBRIÁN, T. J.; MOODY, B. 2002. Mistletoes of North American Conifers. Gen. Tech. Rep. RMRSGTR98. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 123 p.
GLATZEL, G. 1983. Mineral nutrition and water relations of hemiparasitic mistletoes: a question of partitioning. Experiments withLoranthus europaeus onQuercus petraea and Quercus robur. Oecologia, 58(1983), pp.310-32. Recuperado de: https://doi.org/10.1007/BF00379691
GLATZEL, G. y GEILS, B.W. 2009. Mistletoe ecophysiology: host-parasite interactions. Botany 87: 10-15. Recuperado de: https://doi.org/10.1139/B08-096
GONZÁLEZ-TORRALVA. F. L. 2020. Estándares Nutrimentales, Fertilización Química de Juniperus flaccida Schltdl. y nutrición de su huésped parásito Phoradendron juniperinum Engelm. Tesis de Maestría en Ciencias. Colegio de Postgraduados, Texcoco, México. 80 pp.
GRIEBEL, A.; WATSON, D. y PENDALL, E. 2017. Mistletoe, friend and foe: synthesizing ecosystem implications of mistletoe infection. Environmental Research Letters 12: 9 pp. Recuperado de: https://doi.org/10.1088/1748-9326/aa8fff
HOPKINS, B. y ELLSWORTH J. 2005. Phosphorus Availability with Alkaline/Calcareous Soil. Western Nutrient Management Conference Proceedings. Vol. 6. Salt Lake City, UT, USA. pp. 88-93. Recuperado de: https://www.researchgate.net/publication /237734366_Phosphorus_availability_with_alkalinecalcareous_soil
HOSSEINI, S.M.; KARTOOLINEJAD, D.; MIRNIA, S.K.; TABIBZADEH, Z.; AKBARINIA, M. y SHAYANMEHR, F. 2007. The effects of Viscum album L. on foliar weight and nutrient content of host trees in Caspian forests [Iran]. Recuperado de: http://desert.semnan.ac.ir/uploads/kartoli_article7.pdf
INEGI. Instituto Nacional de Estadística Geografía e Informática, 2009. Prontuario de información geográfica municipal de los Estados Unidos Mexicanos. Cardonal, Hidalgo. Clave Geoestadística 13015.
MENGEL, K. y KIRKBY A. E. 1982. Principles of plant nutrition. International Potash Institute. Berna, Suiza. 593 p.
SECRTETARÍA DE MEDIO AMBIENTE Y RECURSOS NATURALES (SEMARNAT). 2002. NOM-021-RECNAT-2000 Que establece las especificaciones de fertilidad, sanidad y clasificación de suelos, estudios, muestreo y análisis. Diario Oficial de la Federación. 31 de diciembre del 2002. 85 p.
OKUBAMICHAEL, D. Y.; GRIFFITHS, M. E. y WARD, D. 2011. Host specificity, nutrient and water dynamics of the mistletoe Viscum rotundifolium and its potential host species in the Kalahari of South Africa. Journal of Arid Environments, 75(10), 898902. Recuperado de: https://doi.org/10.1016/j.jaridenv.2011.04.026
PANVINI, A. D. y EICKMEIER, W. G. 1993. Nutrient and water relations of the mistletoe Phoradendron leucarpum (Viscaceae): how tightly are they integrated? American Journal of Botany, 80 (1993), pp. 872-878. Recuperado de: http://doi.wiley.com/10.1002/j.1537-2197.1993.tb15307.x
RAFTOYANNIS, Y.; RADOGLOU, K.; BREDEMEIER, M. 2015. Effects of mistletoe infestation on the decline and mortality of Abies cephalonica in Greece. Annals ofForest Research 58(1): 1-11. DOI: 10.15287/afr.2015.347. Recuperado de: https://www.afrjournal.org/index.php/afr/article/view/347
RAYA-PÉREZ, J. C.; RAMÍREZ-PIMENTEL, J. G.; COVARRUBIAS-PRIETO, J.; ACEVEDO-LARA, B. y AGUIRRE-MANCILLA, C. 2014. Mineral and Chlorophyll Content of the Psittacanthus calyculatus (DC) G. Don Hemiparasitic Plant and Four Host Trees. Revista Chapingo Serie Ciencias Forestales y del Ambiente, XX (1), 109-117. Recuperado de: http://dx.doi.org/10.5154/r.rchscfa.2013.06.017
REYES-POZO, J. L.; LEÓN-SÁNCHEZ, M. A. y HERRERO-ECHEVERRIA. G. 2018. Efecto de la Fertilización Sobre el Volumen de Madera en Pinus caribaea Morelet var. caribaea Barret y Golfari en Cub. Foresta Veracruzana, 20(2), 29. Recuperado de: https://www.redalyc.org/jatsRepo/497/49758340003/html /index.html
SZMIDLA, H.; TKACZYK, M.; PLEWA, R.; TARWACKI, G. Y SIEROTA, Z. 2019. Impact of common mistletoe (Viscum album L.) on scots pine forests A call for action. Forests 10 (847):1-15. doi:10.3390/f10100847 Recuperado de: https://www.mdpi.com/1999-4907/10/10/847
TENNAKOON K. U.; CHAK W. H. y BOLIN J. F. 2011. Nutritional and isotopic relationships of selected Bornean tropical mistletoehost associations in Brunei Darussalam. Functional Plant Biology 38: 505513. Recuperado de: https://doi.org/10.1071/FP10211
TIMMER, V. y STONE, E. 1978. Comparative Foliar Analysis of Young Balsam Fir Fertilized With Nitrogen, Phosphorus, Potassium, and Lime 1. Soil Science Society of America Journal, Vol. 42, 125-130 pp. Recuperado de: doi:10.2136/sssaj1978.03615995004200010027x
TSCHINKEL, H. 1972. Factores Limitantes del Crecimiento de Plantaciones de Cupressus lusitánica en Antioquia, Colombia. Revista Facultad Nacional de Agronomía Medellín, 27(2), 3-55. Recuperado de: https://revistas.unal.edu.co/index.php/refame/article/view/29961
TÜRE, C.; BÖCÜK, H. y AªAN, Z. 2010. Nutritional relationships between hemi-parasitic mistletoe and some of its deciduous hosts in different habitats. Biologia, 65(5). Recuperado de: https://doi.org/10.2478/s11756-010-0088-5
VÁZQUEZ-CISNEROS, I., PRIETO-RUIZ, J. Á., LÓPEZ-LÓPEZ, M. Á., WEHENKEL, C.,
DOMÍNGUEZ-CALLEROS, P. A. y MUÑOZ-SÁEZ, F. E. 2018. Crecimiento y supervivencia de una plantación de Pinus greggii Engelm. ex Parl. var. greggii bajo diferentes tratamientos de fertilización. Revista Chapingo Serie Ciencias Forestales y del
Ambiente 24(2), 251-264. doi: 10.5154/r.rchscfa.2017.05.036. Recuperado de: https://www.researchgate.net/publication/323970315_Crecimiento_y_supervivencia_de_una_plantacion_de_Pinus_greggii_Engelm_ex_Parl_var_greggii_bajo_diferentes_
tratamientos_de_fertilizacion
VELASCO V. A. 1999. Papel de la nutrición mineral en la tolerancia a las enfermedades de las plantas. Terra Latinoamericana. 17 (3). 192-200. Recuperado de: https://www.redalyc.org/pdf/573/57317303.pdf
WELLS, C.G. y ALLEN, L. 1985. A Loblolly Pine Management Guide: When and Where to Apply Fertilizer (No. SE-GTR-36). U.S. Department of Agriculture, Forest Service, Southeastern Forest Experiment Station, Asheville, NC. Recuperado de: https://doi.org/10.2737/SE-GTR-36
Conflict of interests:
The authors declare not to have any interest conflicts.
Authors' contribution:
The authors have participated in the writing of the work and analysis of the documents.
![]()
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0
International license
Copyright (c) 2020 Fanny Libertad
González-Torralva, Miguel Ángel
López-López, Marcos Jiménez-Casas, Dionicio Alvarado-Rosales