
Revista Cubana de Ciencias Forestales. 2020, January-April 8(1): 75-87
Translated from the original in spanish
Germination of Cinchona officinalis L. seeds in three soils types of Cajamarca, Peru
Germinación de semillas de Cinchona officinalis L. en tres tipos de suelos de Cajamarca, Perú
Germinação de sementes de Cinchona officinalis L. em três tipos de solo em Cajamarca, Peru
Roque Raúl Rodríguez
Barrutia1* ![]() https://orcid.org/0000-0001-5987-8801
https://orcid.org/0000-0001-5987-8801
Israel Barrutia Barreto2 ![]() https://orcid.org/0000-0002-5728-0651
https://orcid.org/0000-0002-5728-0651
Tomás Darío Marín Velásquez3
![]() https://orcid.org/0000-0002-3334-5895
https://orcid.org/0000-0002-3334-5895
1Instituto Nacional de Investigaciones de la Expedición Científica: “Por la ruta del árbol de la Quina”. Perú.
2Centro de Altos Estudios Nacionales. Perú.
3Innova Scientific SAC. Perú.
*Corresponding author: illiary_pakary77@yahoo.es
Received: November 7th, 2019.
Approved: January 21th, 2020.
ABSTRACT
The research analyses the germination of seeds of Cinchona officinalis L., in three soils of populations located in three sectors of the province of Cajamarca in Peru, for its reintroduction in areas where it has disappeared. The seeds were collected in the localities from which the soil samples were obtained and moved to the city of Trujillo, Peru. An experimental design was established per block with three soils and three replicates of 2 kg each with 100 seeds per replicate, for a total of 300 seeds per soil. Perforated plastic containers with lids were used. Spray irrigation was applied daily and the units were covered to conserve humidity. Seedlings were counted between 13 and 60 days and their height was measured at the end of the study. Non-parametric analysis of variance was performed with á = 0.05 to establish the influence of soil on the percentage of germination. It was concluded that the seeds of C. officinalis L. can germinate maintaining similar conditions to those of its habitat (temperature and humidity); also the soil where the biggest quantity of seeds germinated was the one with a sandy loam texture. It was concluded that the type of soil has significant influence on the percentage of germination with p<0.05. It was demonstrated that it can reproduce outside its habitat for reforestation purposes in the areas where this emblematic plant of Peru has disappeared.
Key words: cinchona tree; soil texture; seedlings; reforestation.
RESUMEN
En la investigación se analiza la germinación de semillas de Cinchona officinalis L., en tres suelos de poblaciones ubicadas en tres sectores de la provincia de Cajamarca en Perú, para su reintroducción en zonas donde ha desaparecido. Las semillas se recolectaron en las localidades de donde se obtuvieron las muestras se suelo y se trasladaron a la ciudad de Trujillo, Perú. Se estableció un diseño experimental por bloque con tres suelos y tres réplicas de 2 kg cada una con 100 semillas por réplica, para un total de 300 semillas por suelo. Se utilizaron envases de plástico perforados, con tapa. Se aplicó riego por atomización diariamente y se taparon las unidades para conservar la humedad. El lapso de conteo de las plántulas fue entre 13 y 60 días; además, se midió la altura de las mismas al final del estudio. Se realizó análisis de varianza no paramétrica con á= 0,05 para establecer la influencia del suelo sobre el porcentaje de germinación. Se concluyó que las semillas de C. officinalis L. pueden germinar manteniendo las condiciones similares a las de su hábitat (temperatura y humedad); también el suelo donde germinó la mayor cantidad de semillas fue el de textura franco-arenosa. Se concluyó que el tipo de suelo tiene influencia significativa sobre el porcentaje de germinación con p<0,05. Se demostró que se puede reproducir fuera de su hábitat con fines de reforestación de las zonas de donde ha desaparecido esta planta emblemática del Perú.
Palabras clave: árbol de quina; textura de suelo; plántulas; reforestación.
SÍNTESE
A pesquisa analisa a germinação de sementes de Cinchona officinalis L., em três solos de populações localizadas em três setores da província de Cajamarca, no Peru, para sua reintrodução em áreas onde desapareceu. As sementes foram coletadas nas localidades de onde as amostras de solo foram obtidas e transferidas para a cidade de Trujillo, Peru. Foi estabelecido um desenho experimental por bloco com três solos e três réplicas de 2 kg cada uma com 100 sementes por réplica, para um total de 300 sementes por solo. Foram utilizados recipientes de plástico perfurado com tampas. A irrigação com spray foi aplicada diariamente e as unidades foram cobertas para conservar a umidade. As plântulas foram contadas entre 13 e 60 dias e a sua altura foi medida no final do estudo. A análise não paramétrica de variância com á= 0,05 foi realizada para estabelecer a influência do solo na percentagem de germinação. Concluiu-se que as sementes de C. officinalis L. podem germinar mantendo condições semelhantes às do seu habitat (temperatura e humidade); também o solo onde germinou a maior quantidade de sementes foi aquele com textura arenosa de argila. Concluiu-se que o tipo de solo tem influência significativa na percentagem de germinação com p<0,05. Foi demonstrado que ela pode se reproduzir fora de seu habitat para fins de reflorestamento nas áreas onde esta planta emblemática do Peru desapareceu.
Palavras-chave: árvore cinchona; textura do solo; mudas; reflorestamento.
INTRODUCTION
Cinchona officinalis L., known as the Quina or Cascarilla tree, is a plant belonging to the botanical family Rubiaceae, native to the South American Andean zone, specifically Bolivia, Peru, Ecuador, Colombia and Venezuela (Gómez et al., 2016). From the bark of this tree, a substance rich in an alkaloid called quinine is extracted, which is used to treat malaria. Although it is known generically as officinalis (medicinal), about 23 species of the genus Cinchona are known and Peru is the country that has more species (19), even appears in the National Shield (Alvarez, 2013). Since ancient times, the medicinal use of cinchona was known by the native peoples of the Andes, which made its properties and benefits spread with the Spanish colonization, becoming one of the trees that has saved more lives in the world. Its medicinal use has also been its misfortune, because it is an endangered species due to its excessive exploitation and inadequate use of forests, which has caused the death of thousands of plants (Dulce, 2013). As a tree native to the Andean region, the climate where it is distributed is predominantly warm and humid, with abundant rainfall and cloudiness almost all year round; these are high areas with influence on the microclimate and variations in temperature and rainfall according to altitude and latitude (Zevallos, 1989).
In the case of Peru, the cinchona tree is considered a national symbol, because it appears on the national shield; however, there is a great lack of knowledge among the population about the level of risk of this tree, because it has been indiscriminately extracted from its environment and taken to Asia for commercial exploitation. Although reforestation projects have been carried out with endemic species of the genus Cinchona, such as in the Andean zone of Lambayeque (Muñoz, 2017), some sources, such as that of the news portal Radio Podcast Peru in Lima (RPP, 2017), mention that to date, of the 19 species that exist in Peru, there is no inventory of the trees that are found in the wild.
The reproduction of cinchona requires specific climatic conditions, as it is a species whose habitat is in a cloud mountain area. Therefore, studies have been carried out at laboratory level in order to reproduce it under controlled conditions (Campos-Ruíz et al., (2016); Caraguay-Yaguana, (2016); Jeréz, (2017); Lima et al., (2018), recreating the necessary temperature and humidity conditions at the greenhouse level. The purpose of this research is to determine the germination potential of the plant outside its natural habitat, maintaining similar conditions, for its possible reintroduction in those areas where it has disappeared due to deforestation and overexploitation.
MATERIALS AND METHODS
Seeds of C. officinalis L. were collected from wild populations located in the La Cascarilla-Jaén Minor Settlement (1,850 m), Tayabamba (1,200 m) and Santa Elena (2,100 m). Three composite soil samples were also taken from the same sites where the seeds were collected, following the protocol established in the Peruvian Ministry of Environment's Soil Sampling Guide, (2014).
The seeds and soil samples were moved to the city of Trujillo-Peru, located at sea level, where the trials were conducted, establishing an experimental design by blocks, completely randomized. The nomenclature used for the design is shown in Table 1 (Table 1).
Table 1. - Nomenclature used for the experimental units

The soils were characterized to obtain their texture using the sedimentation method described by Andrades et al., (2015). The soil samples used were 2 kg of soil placed in plastic containers or trays with lids. In each experimental unit, 100 seeds were sown at random according to their origin and watered by atomization, with daily frequency, closing each experimental unit to maintain a microclimate with high humidity that simulated the conditions of the natural environment from which the seeds were obtained.
The monitoring of the number of seedlings in each experimental unit was carried out from 13 days to 60 days, making a total, accumulated and average count, and then, through non-parametric statistical analysis, establishing whether there was a significant difference between germination with respect to the type of soil. Non-parametric statistical models were applied because, as this was a preliminary study, there were not enough data to test assumptions of normality, in which case this type of test is more convenient. The statistical software InfoStat, version 2018, was used. In addition, the height of the seedlings was measured at the end of the maximum research time.
The following parameters, related to seed germination, were also determined (González and Ürozco 1996):
Coefficient of speed of germination (Equation 1).
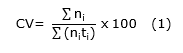
Average germination time (Equation 2).
![]()
Index of germination (Equation 3).
![]()
Seep of germination (Equation 4).
![]()
Where:
ni=number of seeds germinated per day i,
ti=number of days after sowing,
t=germination time from sowing to germination of the last seed,
N=total number of seeds sown,
CV=germination rate coefficient,
T=average germination time,
IG=germination index,
M=germination speed.
RESULTS AND DISCUSSION
The results of the monitoring of the germination of the seeds in each of the experimental units, during a period of 60 days (Table 2).
It is observed that most seeds germinate after 13 days in all soils. On average, 58,1 % of the seeds germinate in that time. It is also evident that in the STY the number of germinated seeds was higher, followed by the SLC and in third place the SSE.
Table 2.- Results obtained from the germination of seeds of C. officinalis L.
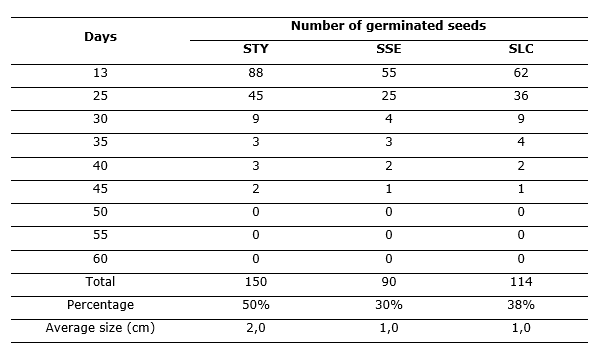
The average size of the seedlings at the end of 60 days of monitoring was higher in STY (2.0 cm) and the average size of the seedlings germinated in SSE and SLC soils was the same (1.0 cm).
The conditions in the locality where the experimental units were established were maintained at a temperature between 13.5 and 22.0 ºC and, as they were managed in a closed environment, the microclimate created presented a humidity close to 100 %, which is common in the areas where the seeds and soil samples were collected. This, according to what Sánchez and Vásquez, (2010) report on the climate map of the department of Cajamarca, where the region of Jaén is classified as an equatorial forest climate, dry, hot and humid, with average temperatures above 18 ºC and no dry season, with rainfall throughout the year (60.85 mm on average), the rainiest months being October, November and December.
The replication of the temperature and humidity conditions present in the natural habitat of C. officinalis L., showed that this species, despite being adapted to the equatorial forest, can germinate, if the humidity condition is maintained, in areas of lower altitude and higher temperatures than its habitat. In this regard, Espinosa and Rios, (2014) in their study of the growth of seedlings of C. officinalis L., reported that they were maintained at an average temperature of 23 º C, which is consistent with the temperature measured in this research.
The percentage of germination of the seeds varied according to the type of soil where they were sown, reaching a maximum of 50 % in the soil of the Tayabamba sector with a sandy loam texture. The percentage of germination obtained coincides with those reported by Lima et al., (2018), which show values between 41.1 and 74.4 %. The results of Caraguay-Yaguana, (2016) were also consistent with those, who obtained 50 % seed germination for low light conditions at the laboratory level Campos-Ruíz et al., (2016) also reported higher germination values than those obtained; however, the cited authors used seeds of Cinchona pubescens and applied treatments to improve the results. Jeréz, (2017) also reported, for seed of C. officinalis L. germination of more than 80 %, but under controlled laboratory conditions, which evidently affects the difference with respect to the results of the present investigation, where the seeds germinated under greenhouse conditions without treatments to improve their germination. It was only sought to simulate the natural conditions of their habitat.
With regard to the growth of the seedlings at 60 days, it was observed that in STY the size exceeded the other soils by 100 %, which showed that not only is there greater germination in this soil, but that the seedlings develop with a greater size in the same period of time compared to the other two soils used. When comparing these results with those reported by Asicona, (2013) it is observed that the development of the seedlings was higher in the present study, due to the fact that the cited author reports a maximum of 3.2 cm at 150 days, so if a linear growth is considered, it would be approximately 1.3 cm at 60 days; similar result to those obtained in the SSE and SLC soil samples, but lower than the STY soil.
The growth rate of C. officinalis L. seedlings was also studied by Espinosa and Rios, (2014), who reported a value of 2.63 cm in 120 days (0.022 cm/day), a result that is also lower than that obtained in the investigation, because the daily growth rate was 0.033 cm/day. The growth rate of cinchona seedlings varies according to the species, which can be seen by comparing the results obtained with works such as that of Gómez et al., (2016), who report that C. pubescens, Vahl (red cinchona) reached a height of between 3 and 4 cm in 60 days, which means a growth rate of between 0.05 and 0.067 cm/day, higher values than those obtained in the investigation for C. officinalis L.
The germination curves stabilize after 40 days, regardless of the type of soil, with cessation of germination after 45 days (Figure 1).
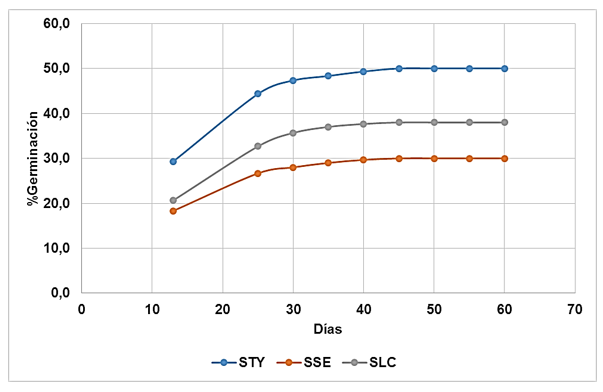
Figure1. - Seed germination curves
The germination curves by type of soil were similar in behavior and uniformity, with a slight difference in the stabilization time in SSE and SLC soils and the results in STY. In their investigation, Moreno and Conde, (2016) determined that the seeds of C. officinalis L. stabilized their germination curve in a minimum time of 50 days, which contrasts with what was obtained in the present investigation, where it was observed that the stabilization occurred five days earlier (45 days), which is due to the different conditions of the trials established for the present investigation, more favorable in comparison with the results of the cited authors.
Another result that contrasts with the one obtained was that of Campos et al., (2016), who obtained germination stabilization times between 23 and 29 days; however, the authors applied treatments to accelerate germination, which is the cause of the differences, since they induced most of the seeds to germinate in a shorter time. In all the cases reviewed, the seeds of C. officinalis L. were germinated under controlled laboratory conditions; most of them were subjected to fertilization treatments, so the research carried out represents a significant contribution and germination was achieved under greenhouse conditions and only with environmental conditions that ensured their development.
According to the tests of normality carried out (Shapiro-Wilks), where results of p-value < 0.05 were obtained, the growth of the seeds does not follow a normal distribution, so for the comparison of the results the test of nonparametric variance of Kruskal-Wallis was carried out. The analysis showed that the percentage of seed germination was significantly influenced by the type of soil with a significance level of 0.05 (Table 3) y (Table 4).
Table 3. - Results of the analysis of variance of Kruskal-Wallis
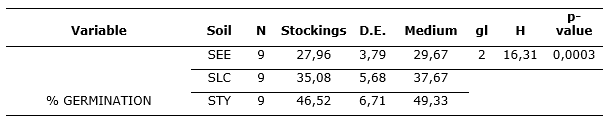
Source: compiled by the authors on the basis of InfoStat results
The statistical results showed that there is dependence between the type of soil and the percentage of germination of the seeds of C. officinalis L. Germination presented significant differences in the soils used in the study. The germination in a soil with a sandy loam texture was also studied by Suárez, (2018), who obtained germination percentages of two varieties of Cinchona between 47.5 and 55.8 %, which is similar to what was obtained in the present investigation (Table 3).
The Table 4 shows that the germination of seeds in soils presents significant differences (p-value < 0.05), which is reflected in the location of each type of soil in different groups, according to the corresponding letter.
Table 4.- Results of Kruskal-Wallis multi-range analysis

Stockings with a common letter are not significantly different (p > 0.05)
Source: compiled by the authors on the basis of InfoStat results
The above suggests that the seeds of C. officinalis need, in addition to suitable climatic conditions, light soils, which have good drainage to germinate in greater proportion. The above was also observed by Mendez et al., (2009), who show that in sandy soil a higher percentage of germination of Psidium guajava L. seeds is obtained, which, although it is not the same species, shows that this type of soil favors germination. Fine grain soils such as SSE and SLC, by retaining water, can influence seed rotting, especially when sown broadcast, because the seeds are directly exposed to irrigation water. The influence of the type of substrate in the germination was observed by Jiménez et al., (2018), who concluded that the influence of the substrate in the germination of seeds of Ochroma pyramidale was statistically significant, which is consistent with what was obtained in the present investigation.
The Table 5 shows the result of soil texture from a granulometric analysis applying sedimentation (Sandoval et al., 2011) to each of the samples (Table 5).
Table 5. - Texture of the soils used
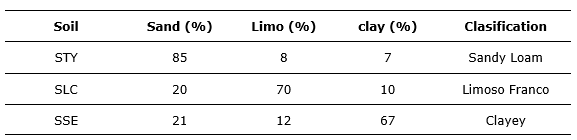
The STY soil is classified as coarse textured (sandy loam) and the SLC and SSE soils are fine textured (Jaramillo, 2002). Therefore, it can be inferred that seeds of C. officinalis L. tend to germinate in greater quantity in the soil of coarse texture; that is, light soils with good drainage. One of the reasons for the lower germination in soils of fine texture is due to the fact that, because they are soils with greater water retention (Ibáñez, 2006), they cause seed rotting, especially in a microclimate of high humidity. The Table 6 shows the results of the germination parameters determined for the interpretation of the same (Table 6).
Table 6. - Calculated germination parameters
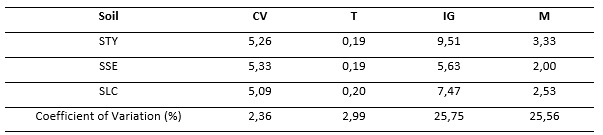
As shown in Table 6, the parameters that show the greatest differences are the germination index and the speed of germination. The speed coefficients and the germination time did not show important variations, which can be observed through the variation coefficient of each parameter, where the variability of the values was less than 5 % for CV and T.
The germination parameters studied show that, in the three soils, the germination speed coefficient and the germination time of the seeds were statistically similar, presenting a variation coefficient of less than 5 %. The parameters, index and speed of germination showed coefficients of variation greater than 5 %, so it is considered that these parameters were influenced by the type of soil where the seeds were sown. The highest index and speed of germination were for the seeds sown in STY, which corroborates what was observed previously and shows that the seeds tend to germinate in greater proportion and with greater speed in the loamy-sandy soil from the Tayabamba sector. With respect to the above, Gómez et al., (2016) concluded that reproduction of the species is possible; however, they clarify that constant irrigation does not substitute for the conditions of the cloud forest, which influences germination and will also depend on the quality of the seeds. Therefore, they recommend reproducing the plants in their habitat, as well as analyzing the relationship between germination and the level of light that the seeds receive.
From the results obtained, it is demonstrated that the seeds of C. officinalis L. can be germinated maintaining the conditions of ambient temperature around 23 ºC and in closed system by atomized irrigation, to maintain the humidity near 100 %.
The soil of the Tayabamba sector was the one with the best properties for seed germination, as a higher percentage was obtained than in the other soil samples, with a significant level of 5 %.
The growth of the seedlings at the end of a 60-day period was satisfactory, reaching a daily growth rate higher than that of other investigations reviewed, with a germination curve that stabilizes at 45 days and a higher germination at 13 days in all soil samples.
Further research should be carried out on the germination and growth of C. officinalis L. outside its natural habitat and under greenhouse conditions, with the aim of achieving a breeding system that will produce seedlings that can be used for the repopulation of areas where it has disappeared.
REFERENCES
ÁLVAREZ ALONSO, José, 2013. El árbol de la calentura. Boletín Instituto Nacional de Salud, vol. 19, pp. 214-215. Disponible en: https://repositorio.ins.gob.pe/handle/INS/296
ANDRADES RODRÍGUEZ, M., MOLINER ARAMENDÍA, A. y MASAGUER RODRÍGUEZ, A., 2015. Prácticas de Edafología. Métodos didácticos para análisis de suelos [en línea]. España: Universidad de La Rioja. ISBN: 978-84-608-5117-2. Disponible en: https://dialnet.unirioja.es/descarga/libro/580696.pdf
ASICONA CABA, P., 2013. Evaluación de cuatro sustratos en semilleros de quina (Cinchona Ledgeriana; Rubiaceae) en Escuintla [en línea]. Tesis Ingeniería en Agronomía. Guatemala: Universidad Rafael Landívar. Disponible en: http://biblio3.url.edu.gt/Tesario/2013/06/17/Asicona-Pablo.pdf.
CAMPOS RUÍZ, J., 2016. Germinación de semillas de quina, Cinchona pubescens Vahl. con ácido giberélico, nitrato de postasio y agua de coco. Pakamuros, vol. 4, no. 1, pp. 8-20. Disponible en: http://revistas.unj.edu.pe/index.php/pakamuros/article/view/38
CARAGUAY YAGUANA, K.A., ERAS GUAMAN, V.H., GONZÁLEZ ZARUMA, D., MORENO SERRANO, J., MINCHALA PATIÑO, J., YAGUANA ARÉVALO, M. y VALAREZO ORTEGA, C., 2016. Potencial reproductivo y análisis de calidad de semillas de Cinchona officinalis L., provenientes de relictos boscosos en la provincia de Loja-Ecuador. Revista Investigaciones Altoandinas, vol. 18, no. 3, pp. 271-280. ISSN: 2306-8582. Disponible en: https://dialnet.unirioja.es/servlet/articulo?codigo=5645611
DULCE MOSTACERO, D., 2013. Medicina mágica: el ayer y hoy del árbol de la Quina. Annalicemos Historia [en línea]. Disponible en: http://annalicemoshist8ria.blogspot.com/2013/06/medicina-magica-el-ayer-y-hoy-del-arbol.html.
ESPINOSA, C.I. y RÍOS, G., 2014. Patrones de crecimiento de Cinchona officinalis in vitro y ex vitro; respuestas de plántulas micropropagadas y de semillas. Revista Ecuatoriana de Medicina y Ciencias Biológicas, vol. 35, no. 1-2, pp. 73-82. ISSN: 0034-9313. Disponible en: https://www.semanticscholar.org/paper/Patrones-de-crecimiento-de-Cinchona-officinalis-in-Espinosa-R%C3%ADos/d99237b1f68a0fed158407e638ac04d9a122b655
GÓMEZ SILVERA, A., BERAUN MACEDO, L.A., GÓMEZ RENGIFO, O.J. y LLATAS DUCEP, E., 2016. Procesos de regeneración natural de la quina o cascarilla (Cinchona spp.): en los bosques de neblina del distrito de Kañaris, región Lambayeque. INIA. Estación Experimental Agraria Vista Florida-Lambayeque [en línea], Disponible en: http://repositorio.inia.gob.pe/bitstream/inia/572/1/Gomez-procesos_reg.pdf.
GONZÁLEZ ZERTUCHE, L. y OROZCO SEGOVIA, A., 1996. Métodos de análisis de datos en la germinación de semillas, un ejemplo: Manfreda brachystachya. Boletín de la Sociedad Botánica de México, vol. 58, pp. 15-30. Disponible en: https://www.semanticscholar.org/paper/M%C3%A9todos-de-an%C3%A1lisis-de-datos-en-la-germinaci%C3%B3n-de-Zertuche-Segovia/83f14cbfc4b71bb5021d62f0b6e85f46ce3c665a
IBÁÑEZ, J.J., 2006. El Agua en el Suelo 4: Textura del Suelo y Propiedades Hídricas. Un Universo invisible bajo nuestros pies. Los suelos y la vida [en línea]. Disponible en: https://www.madrimasd.org/blogs/universo/2006/07/05/33887.
JARAMILLO, D.F., 2002. Introducción a la ciencia del suelo [en línea]. Colombia: Universidad Nacional de Colombia. Facultad de Ciencias Medellín. Disponible en: http://www.bdigital.unal.edu.co/2242/1/70060838.2002.pdf.
JERÉZ BASTIDAS, E.A., 2017. Propagación sexual y asexual de la cascarilla (Cinchona officinalis L.), con fines de potencial reproductivo en el vivero Catiglata del Consejo provincial de Tungurahua [en línea]. Trabajo de Diploma. Ecuador: Escuela Superior Politécnica de Chimborazo. Disponible en: http://dspace.espoch.edu.ec/handle/123456789/7663.
LIMA JIMÉNEZ, N.R., MORENO SERRANO, J.A., ERAS GUAMÁN, V.H., MINCHALA PATIÑO, J., GONZÁLEZ ZARUMA, D., YAGUANA ARÉVALO, M. y VALAREZO ORTEGA, C., 2018. Propagación in vitro de Cinchona officinalis L a partir de semillas. Revista de Investigaciones Altoandinas [en línea], vol. 20, no. 2. ISSN 2313-2957. Disponible en: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S2313-29572018000200002.
MEDIAKIT GRUPO RPP, 2017. El árbol de la Quina: presente en el Escudo, pero casi extinto en el Perú. RPP Lima [en línea]. Disponible en: https://rpp.pe/peru/lima/el-arbol-de-la-quina-presente-en-el-escudo-pero-casi-extinto-en-el-peru-noticia-1067299.
MÉNDEZ NATERA, J.R., MORENO, M.J. y MOYA, J.F., 2009. Efecto de diferentes combinaciones de sustratos (arena, suelo y/o bagazo de caña de azúcar) sobre la germinación de semillas y altura de plantas de guayaba (Psidium guajava L.). Revista Cientifica UDO Agricola [en línea], vol. 9, no. 1. Disponible en: https://www.researchgate.net/publication/47372017_Efecto_de_diferentes_combinaciones_de_sustratos_arena_suelo_yo_bagazo_de_cana_de_azucar_sobre_la_germinacion_de_semillas_ y_altura_de_plantas_de_guayaba_Psidium_guajava_L.
MINISTERIO DEL AMBIENTE, 2014. Guía para muestreo de suelos [en línea]. 2014. S.l.: Dirección General de Calidad Ambiental. Disponible en: http://www.minam.gob.pe/wp-content/uploads/2014/04/GUIA-MUESTREO-SUELO_MINAM1.pdf.
MORENO SERRANO, J.A. y CONDE MONTAÑO, M.E., 2016. Propagación in vivo de Cinchona officinalis L., a partir de material vegetal sexual y asexual, con fines de conservación de la especie [en línea]. Trabajo de Diploma. Ecuador: Universidad Nacional de Loja. Disponible en: https://dspace.unl.edu.ec/jspui/handle/123456789/10270.
MUÑOZ, L., 2017. El árbol de la quina: entre la extinción y su rescate en la zona andina de Lambayeque. Infobosques [en línea]. Disponible en: http://infobosques.com/portal/noticias-y-eventos/el-arbol-de-la-quina-entre-la-extincion-y-su-rescate-en-la-zona-andina-de-lambayeque/.
SÁNCHEZ ROJAS, A. y VÁSQUEZ PERALTA, C., 2010. Mapa climático Departamento de Cajamarca [en línea]. 2010. S.l.: Gobierno Regional de Cajamarca. Disponible en: https://zeeot.regioncajamarca.gob.pe/sites/default/files/MapaClimatico.pdf.
SANDOVAL ESTRADA, M., DÖRNER FERNÁNDEZ, J., SEGUEL SEGUEL, O., CUEVAS BECERRA, J. y RIVERA SALAZAR, D., 2011. Métodos de análisis físico de suelos [en línea]. 2011. S.l.: Sociedad Chilena de la Ciencia del Suelo. Disponible en: http://www.trapananda.uach.cl/proyectos/desarrollo/lib/exe/fetch.php?media=proyectos:metodos_analisis_fisico_suelos.pdf.
SUÁREZ TORRES, J.A., 2018. Caracterización de las semillas de Cinchona capuli L. Andersson y C. lancifolia Mutis y el efecto de las rizobacterias promotoras del crecimiento en la germinación y la formación de plántulas [en línea]. Trabajo de Diploma. Perú: Universidad Nacional Mayor de San Marcos. Disponible en: http://cybertesis.unmsm.edu.pe/handle/cybertesis/8440.
ZEBALLOS POLLITO, P.A., 1989. Taxonomía, distribución geográfica y status del género Cinchona en el Perú [en línea]. Lima: Universidad Nacional Agraria La Molina. Disponible en: https://www.researchgate.net/publication/266558941_Taxonomia_distribucion_geografica_y_status_del_genero_Cinchona_en_el_Peru.
Conflict of interests:
The authors declare not to have any interest conflicts.
Authors' contribution:
The authors have participated in the writing of the work and analysis of the documents.
![]()
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International license.
Copyright (c) 2020 Roque Raúl Rodríguez
Barrutia, Israel Barrutia Barreto,Tomás Darío Marín Velásquez